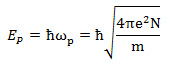分光分析(EELS、EDS、电子构造)
目录
- 1 1. achromatic plane
- 2 2. atom-location by channeling-enhanced microanalysis (ALCHEMI)
- 3 3. alfa filter
- 4 4. ionization energy
- 5 5. ionization cross section
- 6 6. Image EELS
- 7 7. in-column type
- 8 8. Wien filter
- 9 9. escape peak
- 10 10. X-ray emission spectroscopy
- 11 11. X-ray absorption spectroscopy
- 12 12. energy-loss near-edge structure
- 13 13. energy filter
- 14 14. energy resolution
- 15 15. energy analyzer
- 16 16. energy-dispersive X-ray spectroscopy (EDS)
- 17 17. (L2, L3), (M4, M5)…spectra
- 18 18. omega filter
- 19 19. on-site excitation
- 20 20. chemical-bonding state
- 21 21. chemical shift
- 22 22. cathodoluminescence
- 23 23. valence-electron excitation
- 24 24. valence-loss spectrum (low-loss spectrum)
- 25 25. Castaing-Henry filter
- 26 26. absorption effect
- 27 27. absorption-edge energy
- 28 28. Kramers-Kronig relation
- 29 29.Cliff-Lorimer method
- 30 30. fluorescence
- 31 31. k factor
- 32 32. crystal field splitting
- 33 33. atomic-number effect
- 34 34. solid angle
- 35 35. element (elemental) mapping
- 36 36. core-hole interaction
- 37 37. extended energy-loss fine structure (EXELFS)
- 38 38. site occupation (occupancy)
- 39 39. ZAF correction method
- 40 40. sum peak
- 41 41. Gatan imaging filter
- 42 42. density of states
- 43 43. serial detection
- 44 44. oscillator strength
- 45 45. standardless quantitative analysis
- 46 46. spin orbit coupling
- 47 47. three-window method
- 48 48. bremsstrahlung
- 49 49. sector analyzer
- 50 50. zero-loss peak
- 51 51. selection rule
- 52 52. line analysis
- 53 53. occupied state
- 54 54. loss function
- 55 55. onset energy
- 56 56. two-window method
- 57 57. quantitative compositional analysis
- 58 58. electron energy-loss spectroscopy (EELS)
- 59 59. electronic structure
- 60 60. electron prism
- 61 61. electron-probe microanalyzer (EPMA)
- 62 62. point analysis
- 63 63. Drude model
- 64 64. characteristic X-ray
- 65 65. take-off angle
- 66 66. inner-shell (core) excitation
- 67 67. core-loss spectrum
- 68 68. nonisochromaticity
- 69 69. wavelength-dispersive X-ray spectroscopy (WDS)
- 70 70. luminous efficiency
- 71 71. parallel detection
- 72 72. interband transition
- 73 73. band gap
- 74 74. peak-to-background ratio
- 75 75. optical absorption spectrum
- 76 76. unoccupied state
- 77 77. finger printing
- 78 78. Fermi level
- 79 79. depth profile
- 80 80. impurity level
- 81 81. plasma oscillation
- 82 82. plasmon
- 83 83. analytical electron microscopy (AEM)
- 84 84. analysis region
- 85 85. Bethe Ridge
- 86 86. post-column type
- 87 87. Moellenstedt analyzer
- 88 88. area analysis
- 89 89. monochromator
- 90 90. dielectric function
- 91 91. retarding
- 92 92. continuous X-ray
- 93 93. Rowland circle
- 94 94. Lorentz model
- 95 95. live time
- 96 96. process time
- 97 97. volume plasmon
1. achromatic plane
Let electron beams with different energies, which exit from one point on the object plane and pass through an energy filter, meet at one point on an image plane without energy dispersion. This image plane, on which chromatic aberration does not appear, is called the "achromatic plane." An image formed on the achromatic plane is called the achromatic image.
Related term chromatic aberration
2. atom-location by channeling-enhanced microanalysis (ALCHEMI)
"Atom-location by channeling-enhanced microanalysis (ALCHEMI)" is a method that determines sites (locations) of impurity atoms in a crystal by utilizing a phenomenon where the incident electrons pass through specific atom sites (electron channeling). Sites occupied by impurity atoms can be distinguished from the intensity difference of characteristic X-rays (EDS spectra) observed at a small positive tilt angle and a small negative tilt angle from an exact Bragg condition, the tilt angle being adjusted by observing the electron diffraction pattern. In this method, EDS spectra are measured at two crystal orientations but recently, a more reliable technique has been prevailing. That is, a technique of two-dimensional rocking of the incident electron beam enables us to obtain patterns of characteristic X-ray intensities over a large angular range.
Related term characteristic X-ray, electron channeling, site occupation (occupancy)
3. alfa filter
One of the in-column type energy filters, which is installed between the intermediate and projector lenses in a TEM. The "alfa filter," whose principle of energy dispersion is the same as that of the omega filter, consists of two electromagnets instead of four electromagnets in the omega filter. Since the trajectory of electrons passing through the alfa filter takes the shape of a letter "alfa," this is called alfa filter. Its energy dispersion is ~0.7 μm/eV, which is smaller than that of the omega filter.
Related term energy filter, omega filter
4. ionization energy
An energy required for removing one electron from an atom at the ground state to make a monovalent cation. It is called the first ionization energy.
5. ionization cross section
When a neutral atom or molecule loses or receives electrons by collision with other particles, the atom or molecule becomes an ion. This phenomenon is called ionization. "Ionization cross section" is the ionization probability expressed by dimension of area.
6. Image EELS
"Image EELS" means a TEM image formed by the electrons of a specific energy selected from an EELS spectrum (electron energy-loss spectrum).
Related term element (elemental) mapping, energy filter
7. in-column type
An energy filter or an energy analyzer that is installed in the column of a TEM is classified as "in-column type." This type includes an omega filter and an alfa filter.
Related term alfa filter, omega filter, post-column type
8. Wien filter
One of the in-column type energy filters. The "Wien filter" uses magnetic and electric fields perpendicular to each other for getting energy dispersion, though the omega filter and the alfa filter use only magnetic fields. The Wien filter is used as a monochromator by incorporating it in the illumination system of a TEM. Since its energy dispersion for a high-voltage 200 kV electron beam is small, the electron beam is introduced into the filter before the beam is accelerated or after the beam is decelerated to several 100 V. In this case, its energy dispersion is ~10 μm/eV. There is a limitation on applying a high voltage to the Wien filter due to discharge between electrodes. Thus, the filter is used for an incident electron beam at a voltage of ~10 keV or less. The advantage of the Wien filter is that the electron trajectory in the filter is parallel to the optical axis or forms a straight line.
Related term energy filter, omega filter, alfa filter, monochromator
9. escape peak
In EDS analysis, when characteristic X-rays emitted from a specimen are detected with a semiconductor detector, part of the energies of the X-rays entering the detector is used to excite inner-shell electrons of silicon (Si) that is a constituent element of the detector. As a result, a small peak appears in an EDS spectrum at an energy lower than that of characteristic X-rays by the excitation energy of Si. This peak is called an "escape peak" and therefore, care must be taken for spectral analysis.
Related term sum peak
10. X-ray emission spectroscopy
A spectroscopy method, which measures the density of states of the occupied states (valence band) by analyzing X-rays emitted from a substance illuminated with an X-ray beam or an electron beam.
Related term energy-dispersive X-ray spectroscopy, EDS, wavelength-dispersive X-ray spectroscopy, WDS, density of states
11. X-ray absorption spectroscopy
A spectroscopy method to acquire a spectrum of X-rays absorbed by a substance. In a similar manner as EELS, XAS of the soft X-ray region enables us to obtain the density of states of the unoccupied state (conduction band) of a material.
Related term electron energy-loss spectroscopy, EELS, optical absorption spectrum, electromagnetic wave
12. energy-loss near-edge structure
A fine structure appearing in an energy region of about 30 eV above the absorption-edge energy in the EELS core-loss spectrum. This structure is called "energy-loss near-edge structure (ELNES)." The ELNES is produced by the transitions of electrons from the inner-shell state to the conduction band (unoccupied state), enabling us to obtain the density of states of the conduction band of a substance.
Related term core-loss spectrum
13. energy filter
Instrument that selects electrons with only specific energies in electrons exiting from a specimen. When only elastically scattered electrons are selected by the energy filter, the background due to inelastically scattered electrons is successfully removed. Thus, a clear microscope image or diffraction pattern is obtained, which provides high-precision structural information. When the absorption-edge energy of a specific element is selected, the corresponding element is mapped. The energy filter is classified into the incolumn type incorporated in the TEM column and the post-column type attached below the column. There are various energy filters with different designs.
Related term elastically scattered electron, inelastically scattered electron, element (elemental) mapping, omega filter, alfa filter, Wien filter, Castaing-Henry filter
14. energy resolution
"Energy resolution" is the minimum energy (eV) of a spectral peak that can be resolved in spectroscopy. In EDS, the detector performance determines the energy resolution to be 130 to 140 eV. In WDS, it is about 10 eV; however recently, some WDS analyzers can produce an energy resolution below 1 eV. In EELS, the energy spread of the incident electron beam almost determines the energy resolution. Standard EELS resolution using a field-emission electron gun is about 0.7 eV. When an electron gun is equipped with a monochromator, a higher energy resolution of about 0.2 eV is obtained.
Related term field-emission electron gun, FEG, monochromator, energy-dispersive X-ray spectroscopy, EDS, wavelength-dispersive X-ray spectroscopy, WDS, electron energy-loss spectroscopy, EELS
15. energy analyzer
An energy analyzer is used to disperse energies of characteristic X-rays or soft X-rays in WDS, and those of inelastically scattered electrons in EELS. In the case of WDS, analyzing crystals and gratings are respectively used for characteristic X-rays (element analysis) and for soft X-rays (measurements of the density of states of the valence band). In the case of EELS, an omega filter is used as an incolumn type energy analyzer, and a sector analyzer is used as a post-column type energy analyzer.
Related term omega filter, sector analyzer, Moellenstedt analyzer, analyzing crystal, grating
16. energy-dispersive X-ray spectroscopy (EDS)
Energy-dispersive X-ray spectroscopy (EDS) is an element analysis method. Characteristic X-rays generated from a specimen are detected by a semiconductor detector and converted into electric signals. In the EDS analyzer, the pulse currents that are proportional to the energies of the detected characteristic X-rays are generated, and then these currents are measured with a multi-channel pulse-height analyzer. EDS has higher detection efficiency of X-rays than WDS, but the analyzing power of light elements is lower than WDS. (EDS cannot analyze elements from boron (B) on down.) Since the illumination current of the electron beam for EDS can be decreased from several pA to several nA compared with WDS, the beam damage to a specimen is small. Normally, the resolution of EDS is ~140 eV for Mn Kα emission at 5.9 keV. The resolution determined by statistical counting error is around the square root of energy E of the generated X-ray × √3. (The number of created electrons n is obtained as n~E/3 by taking the band gap energy as ~3 eV. Since the statistical error Δn is ~√n, the energy spread (error) is obtained to be ~Δn・3 = √E・√3.) Recently, an EDS detector that can resolve a beryllium (Be) peak has been developed. Its quantification accuracy is 0.5 to 5%. Compared with EPMA that uses analyzing crystals, EDS provides high spatial resolution, 100 times better than EPMA but shows 10 times worse quantification accuracy than EPMA. "EDX" is also used as the abbreviation of energy-dispersive X-ray spectroscopy.
Related term characteristic X-ray, semiconductor detector (solid-state detector), SSD, escape peak, sum peak, wavelength-dispersive X-ray spectroscopy, WDS
17. (L2, L3), (M4, M5)…spectra
Absorption edges characteristic of elements, which appear in an energy region higher than 50 eV in an EELS spectrum. These absorption edges arise due to excitations of inner shell electrons to the conduction band. They are called "K, L, M…" shell excitation spectra depending on the excited inner shell. The inner-shell levels have fine structures due to the spin orbit coupling. The split levels are expressed as K(1s1/2), L1(2s1/2), L2(2p1/2), L3(2p3/2), M1(3s1/2), M2(3p1/2), M3(3p3/2), M4(3d3/2), M5(3d5/2), …. Since the difference of the L2 & L3 energy levels of 3d transition metals is 5 to 20 eV, two spectra with similar shape successively appear with the energy difference in the EELS spectrum. For Si and Al, the L2 & L3 spectra form a non-separated absorption-edge spectrum because the energy separation of the L2 & L3 levels is 1 eV or less. Thus, they are denoted as L2,3. The intensity ratio of the L2 & L3 spectra is expected to be 1:2 from the occupation ratio of the levels. However, the ratio experimentally observed is different from the ratio expected due to non-flat configuration of the conduction band and the core-hole interaction. In the case of the excitation of M shell electrons of 4d transition metals, M4 and M5 spectra appear successively with an energy difference of 2 to 10 eV.
Related term electron energy-loss spectroscopy, EELS, core-loss spectrum, spin orbit coupling, core-hole interaction
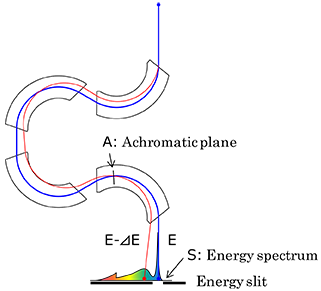
18. omega filter
One of the in-column type energy filters, which is installed between the intermediate and projector lenses in a TEM. The filter (spectrometer) is composed of four electromagnets and has a shape of the letter “omega.” Thus, this is called “omega filter.” Its energy dispersion is approximately 1 μm/eV for a 200 kV electron beam. The omega filter is used to date to acquire filtered (zero-loss) images, energy-loss images and zero-loss CBED patterns.
Schematic of the action of the omega filter and an acquired spectrum.
A blue curve shows the trajectory of electrons passing through the optical axis with an energy E. A red curve shows the trajectory of electrons suffered by energy dispersion with an energy loss of ⊿E. When the optical plane at which energy dispersion (S) is created is projected onto the screen, the intensity distribution against energy losses, or an energy spectrum is observed.
When the achromatic plane (A), at which energy dispersion disappears, is projected onto the screen, an image without energy dispersion is observed. In this case, if the energy slit placed on plane S is inserted to pass no energy-loss electrons, a zero-loss image (so called “filtered image”) is acquired. If the energy slit selects energy-loss electrons, an energy-loss image is obtained.
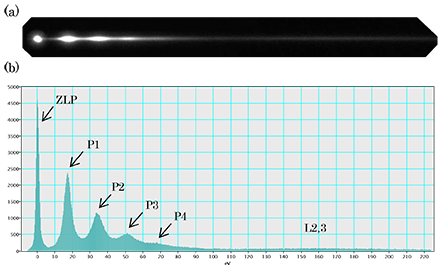
(a) Energy spectrum of Si acquired at an accelerating voltage of 200 kV. (b) Line profile of the energy spectrum. ZLP is the zero-loss peak. P1 is a peak of a plasmon loss electrons (Ep = 16.7 eV). P2, P3… are peaks produced by multiple scattering of the plasmon. Weak and broad peaks L2,3 are the spectra due to excitation of inner-shell electrons.
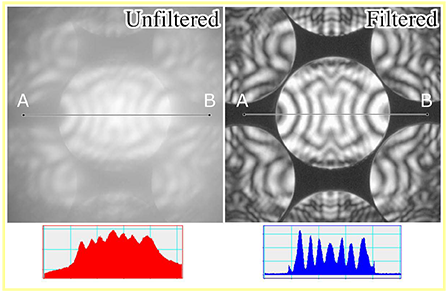
CBED patterns of cubic-BN [110] acquired at an accelerating voltage of 100 kV. The two patterns were taken by projecting the CBED patterns formed at the achromatic plane onto the screen. (a) CBED pattern without using the energy slit (unfiltered pattern). (b) Filtered CBED pattern by selecting zero-loss energy with the energy slit. The graphs below the patterns are line profiles of each line A-B.
In the unfiltered pattern at the left, the patterns inside the CBED disks are unclear. To the contrary in the filtered pattern at the right, where the lost energies approximately more than 10 eV are cut, the patterns in the disks are clearly seen. The energy filter is indispensable for quantitative analysis of CBED patterns.
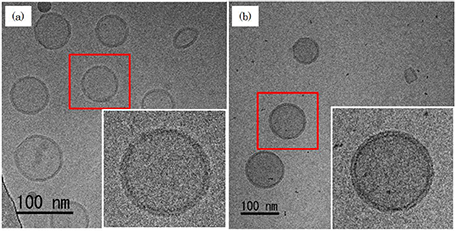
TEM images of ice-embedded liposome acquired at an accelerating voltage of 200 kV, taken by projecting the images formed at the achromatic plane onto the screen. (a) Conventional image without using the energy filter (unfiltered image). (b) Zero-loss image using the energy filter (filtered image).
It is seen that the image contrast of liposome is enhanced in the filtered image (b).
Related term energy filter, alfa filter, Wien filter, Castaing-Henry filter, achromatic plane
19. on-site excitation
In EELS, "on-site excitation" means that the inner-shell excitation occurs spatially only on an excited atom. When excitation of an inner-shell electron to an unoccupied state (band) is considered, since the wavefunction of the inner-shell electron is localized on an atom, if the final state is not overlapped with that wavefunction, the excitation (transition) does not occur. Thus, the ELNES spectrum reveals the anti-bonding state of the excited atom.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES
20. chemical-bonding state
"Chemical-bonding state" means the energy and momentum of electrons which connect atoms to each other in a molecule or crystal. EELS enables us to perform detailed analysis of the chemical-bonding state.
Related term electron energy-loss spectroscopy, EELS
21. chemical shift
"Chemical shift" in solid state physics means that the energy level of an inner-shell electron changes when the valence number (chemical state) changes. For example, if one valence electron is removed from an atom, inner-shell electrons are further attracted to the nucleus of the atom and the energy level of the inner-shell electrons shifts to a lower level. Thus, the energy difference between the inner-shell level and the bottom of the conduction band becomes larger. As a result, the onset energy of the EELS core-loss spectrum shifts to a higher energy. The removal of one valence electron can cause an energy shift of about 2.5 eV.
Related term core-loss spectrum
22. cathodoluminescence
Electrons in a solid are excited by electron-beam irradiation leaving holes. The electrons recombine with the holes to emit light (ultraviolet to infrared). This phenomenon is called "Cathodoluminescence". Cathodoluminescence is utilized as a method to analyze the electronic structure of solids in an electron microscope. The method can measure local electronic states (energy levels) of impurities and defects, the electronic states being formed between the valence band and the conduction band (in the forbidden band). Thus, the method enables the evaluation of inorganic materials containing the structural defects with a high spatial resolution (better than 1 μm) by the combined use with structural information obtained from SEM / STEM / TEM images. Applications to biological specimens, such as detection of specific proteins, have also been studied.
In the case of semiconductors, electrons in the valence band are excited to the conduction band by incident electrons with generating holes in the valence band (referred to as electron-hole pair generation). The excited electrons and holes form a pair (free exciton (FX): It occurs remarkably at low temperatures) bound by Coulomb force. Free excitons are unstable and recombine at arbitrary places and emit light. At room temperature or higher temperatures, the free excitons cannot be formed, thus the generated electrons and holes diffuse independently in the semiconductor as carriers. When they are trapped by impurity atoms creating donors and acceptors, radiative recombination occurs. When they are captured by lattice defects such as dislocations, non-radiative recombination occurs. For example, the distribution of impurity atoms can be detected from a CL image using a specific wavelength.
CL measurements of semiconductors have to be conducted at liquid nitrogen temperature or lower because the luminescence intensity weakens due to the increases of non-radiative recombination through the lattice vibrations. Also, the CL measurements have to be performed with an accelerating voltage less than 100 kV. : When the accelerating voltage of the incident electron beam exceeds the threshold voltage (approx. 100 kV), the generation of point defects increases. The point defects create deep defect levels within the forbidden band and non-radiative recombinations via those levels increase, causing decrease of the luminescence intensity.
In the case of insulators, impurity centers are formed by d-electrons of transition elements and f-electrons of rare earth elements when they are added in oxides or sulfides, and color centers are formed at vacancies in alkali halide crystals. These electrons trapped at such localized centers produce a ground level and an excited level within the forbidden band. Light emission occurs at the electronic transition between those levels, enabling positional information on additive elements to be obtained. Restriction on the accelerating voltage of the incident electron beam is not severe.
In the case of organic materials, light is emitted by electronic transition from the lowest unoccupied molecular orbital (LUMO) of the organic molecule to the highest occupied molecular orbital (HOMO). Local information cannot be obtained, but information on degradation and aging of organic molecules can be obtained. Since the organic specimen is susceptible to electron-beam irradiation, observation at a low accelerating voltage is required. (By Naoki Yamamoto in Tokyo Institute of Technology)
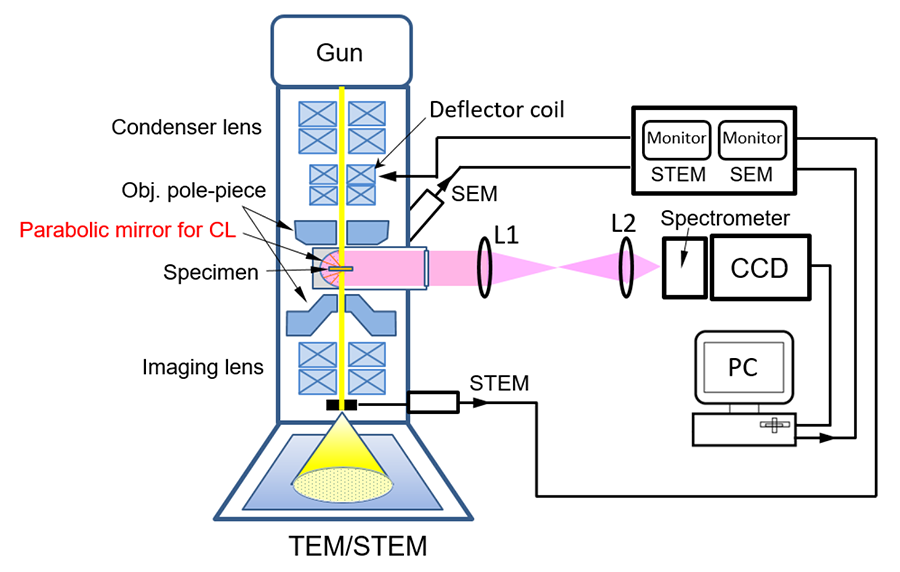
Fig.1 Schematic diagram of cathodoluminescence (CL) detection system. Light emitted from a specimen in a STEM (SEM) by an incident electron beam is collected and reflected by a parabolic mirror, and becomes parallel light, then guided outside the electron microscope. This light passes through the lens 1 (L1) and is focused by the lens 2 (L2) onto the slit of the spectrometer in front of the CCD detector. The spectrum of the light passing through the slit is recorded with the CCD. The incident electron beam is two-dimensionally scanned using the beam deflector controlled with a computer, and the emission spectra from each beam position are successively acquired by the CCD. After measurement, a two-dimensional monochromatic CL image is displayed by selecting a specific wavelength. From the STEM or SEM image and the CL image of the specimen, the optical properties of the structural defects are obtained.
The luminescence ranges from infrared light (1 to 2.5 μm in wavelength) to ultraviolet light (200 nm to 380 nm). Specific gratings and detectors are used for ultraviolet-, visible- and infrared-light depending on the wavelength range. The wavelength resolution of the detector is approximately 1 nm (less than 10 meV in energy).
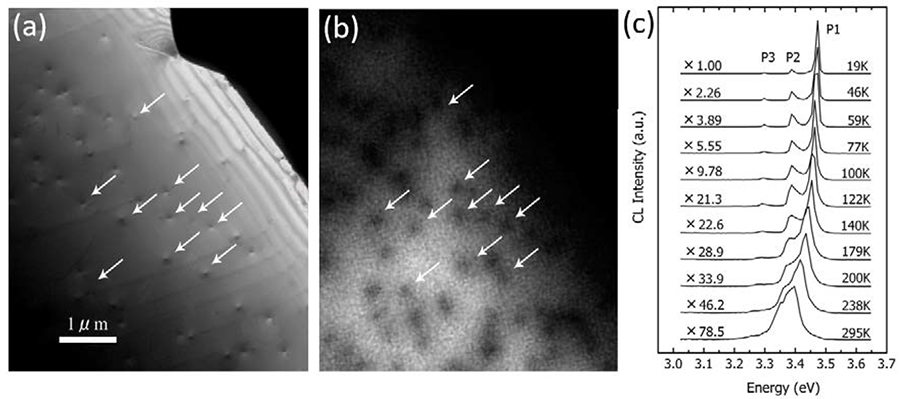
Fig.2 TEM dark field image, CL image and CL spectra of GaN epitaxial film containing the dislocations penetrating the specimen (accelerating voltage 80 kV) A GaN epitaxial film grown by the MOCVD (metal organic chemical vapor deposition) method on a sapphire substrate (film thickness 4 μm) was thinned from the substrate side by ion milling. A transmission electron microscope (TEM) image and a CL image and CL spectra of a thin edge region containing the dislocations penetrating the specimen are shown.
(a) TEM dark field image. Arrows in the image indicate the dislocations.
(b) Monochromatic CL image of the same area in (a) taken at room temperature using an emission light with wavelength of 336 nm (3.39 eV).
It is seen that free exciton (FX) emission occurs in the whole area of the specimen (bright part). Dark contrast appears at the dislocations because the carriers are captured there and non-radiative recombination occurs.
(c) CL spectra measured at different specimen temperatures. The main peak (P1) is FX emission of GaN, the P2 peak is light emission associated with impurities, and the P3 peak is donor-acceptor pair emission (D, A). The FX emission intensity, which is important for elucidating the dislocation positions, decreases with increasing temperature, i.e., the intensity at room temperature is two orders of magnitude smaller than that at 19K. Thus, measurement at low temperature is necessary, especially for a thin specimen with a low emission intensity. From the analysis of the CL intensity distribution around a dislocation, the diffusion length of the carrier can be measured.
Related term electronic structure analysis
23. valence-electron excitation
A phenomenon in which valence electrons are excited to the conduction band. EELS enables us to obtain the dielectric function of a substance through the valence-loss spectrum (low-loss spectrum).
Related term valence-loss spectrum (low-loss spectrum)
24. valence-loss spectrum (low-loss spectrum)
A spectrum in the low energy region (below ~50 eV) in an EELS spectrum is called "valence-loss spectrum (low-loss spectrum)." This spectrum enables the band gap energy (0 to 10 eV) and the plasmon energy (10 to 50 eV) to be obtained. Dielectric and optical properties of solids can be investigated from the spectrum.
Related term electron energy-loss spectroscopy, EELS, interband transition, band gap, plasma oscillation, plasmon, electronic structure analysis
25. Castaing-Henry filter
One of the in-column type energy filters, which is installed between the intermediate and projector lenses in a TEM. The filter consists of an isosceles-triangle electromagnet and an electrostatic mirror that reflects incident electrons to reverse their traveling direction. Since the "Castaing-Henry filter" exerts an electrostatic potential, the incident-electron energy is limited to 80 keV. Its energy dispersion is ~1 μm/eV for a 80 kV electron beam.
Related term energy filter, omega filter, alfa filter, Wien filter
26. absorption effect
In spectroscopic analysis of characteristic X-rays (EDS), the "absorption effect" means that part of X-rays generated within a specimen is absorbed by the specimen. Since this effect cannot be neglected when a specimen is thicker (~several 10 nm or thicker though depending on target elements), correction for the detected X-ray intensity is required in quantitative analysis. The correction of the absorption effect is crucial for thick specimens because this effect is larger than the atomic-number effect and the fluorescence excitation effect.
Related term ZAF correction method, atomic-number effect, fluorescence excitation effect
27. absorption-edge energy
The energy to excite an electron bound to an orbit of an atom to the lowest level of the unoccupied state.
28. Kramers-Kronig relation
A formula that gives the relation between the real and imaginary parts of the response function in linear response theory. In EELS, "Kramers-Kronig relation" is used when obtaining a dielectric function from the loss function of a substance.
Related term valence-loss spectrum (low-loss spectrum), loss function
29.Cliff-Lorimer method
The Cliff-Lorimer method is a qualitative measurement method of elements in spectroscopic analysis of characteristic X-rays (EDS). It is also called the thin-film approximation method. The method is applied when a specimen thickness is 10 nm or less (though depending on measured elements). For example, when a substance is composed of two elements A and B, characteristic X-ray intensities IA and IB are measured. Then, the concentration ratio of element A to element B (CA/CB) is obtained from equation CA/CB = k・IA/IB, where k is a proportionality factor, which is determined by ionization cross sections, fluorescent yields etc. of the elements. If a specimen is thin, quantitative measurements can be performed with a relatively high accuracy even when corrections for the atomic-number effect, the absorption effect and the fluorescence excitation effect are neglected. On the other hand, if a specimen is thick, the corrections must be executed for the measured intensities (ZAF correction).
Related term k factor, atomic-number effect, absorption effect, fluorescence excitation effect, ZAF correction method
30. fluorescence
30-1. fluorescence
"Fluorescence" means light (electromagnetic wave; infrared ray to ultra violet ray to X-ray) emitted from electronically excited states which are created by absorption of incident X-rays, electrons etc. In EDS, fluorescent X-rays are used for element analysis. In a crystal, an electron in the K-shell of an element is excited to an unoccupied state, and then an electron in the L-shell of the element falls to the K-shell, characteristic K X-rays inherent to the element being emitted.
Related term energy-dispersive X-ray spectroscopy, EDS
30-2. fluorescence excitation effect
In spectroscopic analysis of characteristic X-rays (EDS), the "fluorescence excitation effect" means that X-rays emitted from non-target elements, whose energy is higher than that of the characteristic X-rays of the target element, are absorbed by the target element and characteristic X-rays of the target element are additionally emitted. Since this effect cannot be neglected in quantitative analysis when a specimen is thicker (~several 10 nm or thicker though depending on target elements), correction for the detected X-ray intensity is required. This effect is, however, smaller than the absorption effect.
Related term ZAF correction method, absorption effect, atomic-number effect
31. k factor
A factor used for the Cliff-Lorimer method in EDS analysis. For the determination of the k factor, a substance composed of two elements A and B, whose composition is similar to the target substance, is used as a standard specimen. Characteristic X-ray intensities IA and IB of the standard specimen are measured. And then the k factor is determined by equation k = CA/CB・IB /IAusing the known compositions CA and CB. The k factor is theoretically given by equation k = (MAQBωBαB)/(MBQAωAαA), where M, Q, ω and α are atomic weight, ionization cross section, fluorescent yield and the ratio of Kα line to the total K lines of a substance, respectively. It is noted that the accuracies of Q and ω are low. In actual cases, the absorption due to the window material is also needed to be taken into account. For the elements up to 3d metals, it is said that the error between the quantitative analysis using experimental k factor and that with the theoretical k factor is about 10%. For a substance composed of elements with greatly different atomic numbers, the accuracy of the analysis is low.
Related term Cliff-Lorimer method, standardless quantitative analysis
32. crystal field splitting
"Crystal field splitting" means that the energy level of 3d electrons is split depending on a crystal field surrounding the 3d electrons. In the case of the perovskite structure, a transition metal having 3d electrons is located at the center of the oxygen octahedron, the energy of the eg orbital of the 3d electrons, which is faced to the oxygen atoms, is higher than that of the t2gorbital, which is pointing between the oxygen atoms. In an EELS spectrum, two peaks corresponding to the t2g and eg states due to the crystal field splitting appear when 2p electrons are excited to the 3d unoccupied band and when 1s electrons are excited to the 2p band hybridized with the 3d state.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES
33. atomic-number effect
In spectroscopic analysis of characteristic X-rays (EDS), the "atomic-number effect" means that, since the amount of incident electrons which do not contribute to the emission of the characteristic X-rays of a target element (the excitation of the electrons of the target element) due to backscattering of the incident electrons is dependent on the average atomic number, the intensity of the X-rays generated from the specimen is dependent on the atomic numbers of the constituent elements of the specimen. When the atomic numbers of constituent elements are largely different, this effect has to be considered. Since this effect cannot be neglected in quantitative analysis when a specimen is thicker (~several 10 nm or thicker though depending on measured elements), correction for the detected X-ray intensity is required.
Related term ZAF correction method, absorption effect, fluorescence excitation effect
34. solid angle
"Solid angle" for detection of EDS signals is a three-dimensional angle formed by the cross-sectional detection area (sphere) of the detector with respect to the incidence point of the electron beam on the specimen from which characteristic X-rays are emitted. The solid angle can be made larger for a larger detection area and a smaller distance between the specimen and the detector.
Related term energy-dispersive X-ray spectroscopy, EDS
35. element (elemental) mapping
"Element (elemental) mapping" is carried out by using EELS spectra and EDS spectra. In the case of EELS mapping, the loss energy characteristic of each element in a core-loss spectrum is selected with the energy slit at the EELS mode, and then the mapping of the element is obtained by switching to the image mode. (This explanation is based on TEM-EELS but there is STEM-EELS method which uses the scanning technique like EDS mapping.) In the case of EDS mapping, the X-ray intensities characteristic of each element are measured while the electron beam is two-dimensionally scanned on the specimen, and then brightness modulations corresponding to the X-ray intensities are displayed on a computer monitor synchronized with the scanning signals. As a result, a two-dimensional distribution image of an element is obtained.
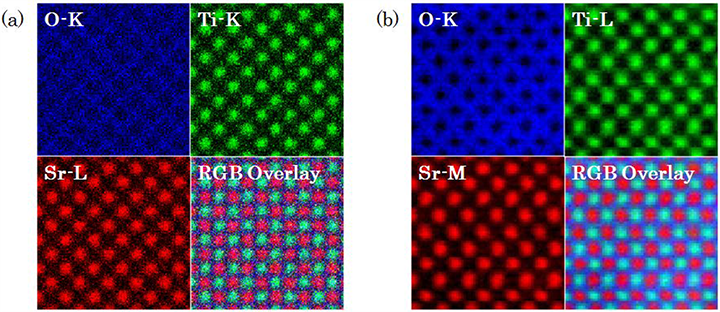
(a) High spatial resolution elemental maps acquired by EDS. Specimen: SrTiO3, Accelerating voltage: 80 kV. X-ray spectra are acquired by two-dimensionally scanning the electron beam over the specimen, and intensities of characteristic X-rays of O-K, Ti-K and Sr-L are displayed on the corresponding scanning points. RGB map is the overlay of each elemental map.
(b) High spatial resolution elemental maps acquired by STEM-EELS. Specimen: SrTiO3, Accelerating voltage: 80 kV. Core-loss spectra are acquired by two-dimensionally scanning the electron beam over the specimen, and intensities of energy-loss electrons of O-K, Ti-L and Sr-M are displayed on the corresponding scanning points. RGB map is the overlay of each elemental map.
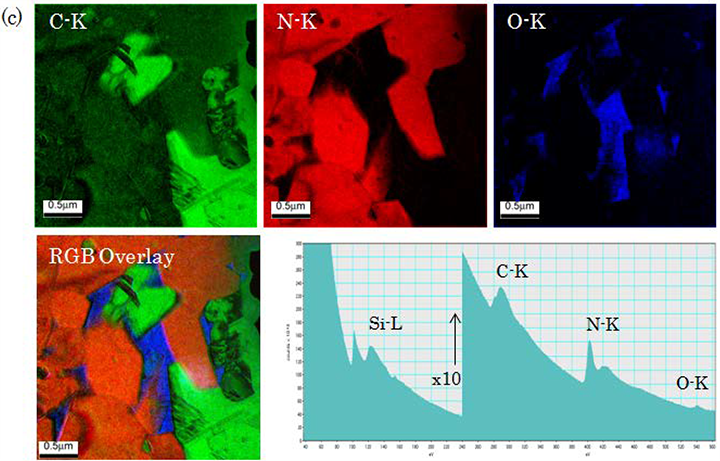
(c) Elemental maps acquired by TEM-EELS using an Omega filter. Specimen: SiC/Si3N4, Accelerating voltage: 1250 kV. Core-loss spectra (bottom-right figure) are acquired by TEM-EELS. Energy-loss intensities of C-K, N-K or O-K which are characteristic of respective elements are chosen with the energy selection slit. Then, the energy filtered images of the elements C, N and K are displayed as elemental maps. RGB map is the overlay of each elemental map. Elemental mapping using TEM-EELS enables to acquire a wide-field low-magnification elemental map image over an area of a few μm.
Related term core-loss spectrum, energy filter, high-angle annular dark-field scanning transmission electron microscopy, HAADF-STEM
36. core-hole interaction
A Coulomb interaction between a hole created in an inner shell and an excited electron when an inner-shell electron is excited. In the case of metals, "core-hole interaction" is small because the free electrons screen the effect of the hole, but in the case of oxides having no free electrons, this interaction is large. In EELS, the core-hole interaction causes the absorption edge to shift to a lower energy side. If the energy difference between the excited electron and the hole is small, the interaction is large. For example, the energy shift is 1.4 eV (calculated to be 1.7 eV) for B1s→2p excitation of hex. BN. When an ELNES spectrum is calculated by taking account of the core-hole interaction, the spectrum shows much better agreement with the experimental spectrum up to about 10 eV from the absorption edge.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES
37. extended energy-loss fine structure (EXELFS)
A fine structure appearing in an energy region of about 40 to 200 eV above the absorption-edge energy in the EELS core-loss spectrum. This structure is called "extended energy-loss fine structure (EXELFS)." The EXELFS is produced due to the scattering of electrons, which are excited from the inner-shell state to the conduction band (unoccupied state), by the adjacent atoms. When the EXELFS spectrum is Fourier-transformed, information on local atomic arrangements is obtained. EXELFS corresponds to EXAFS in X-ray spectroscopy.
Related term core-loss spectrum
38. site occupation (occupancy)
"Site occupation (occupancy)" means that atoms occupy specific sites. This term is particularly used in ALCHEMI to determine what impurity atoms or doped atoms occupy what sites.
Related term ALCHEMI
39. ZAF correction method
In spectroscopic analysis of characteristic X-rays, the "ZAF correction method" is used for quantitative analysis of target elements. As a specimen is thicker (~several 10 nm or thicker though depending on measured elements), the intensity of the emitted characteristic X-rays is influenced by the atomic-number effect, the absorption effect and the fluorescence excitation effect. Corrections of these three effects are required. In actual correction, the relative X-ray intensities obtained from an unknown specimen against those from a standard specimen (usually a compound of a simple composition) are measured. And then, the corrections of the three effects are made to the relative intensities. In the case of EPMA, the three effects are corrected. On the other hand, in the case of TEM, only the absorption effect, which has the largest effect, is taken into account in many cases. Normally, the Cliff-Lorimer method (thin-film approximation method) for a thin specimen is applied, which often provides good quantitative results with a relatively high accuracy.
Related term energy-dispersive X-ray spectroscopy, EDS, atomic-number effect, absorption effect, fluorescence excitation effect, Cliff-Lorimer method
40. sum peak
In EDS analysis, characteristic X-rays emitted from a specimen are detected with a semiconductor detector. Pulse voltages that are proportional to the energies of the detected characteristic X-rays are generated, and then these voltages are measured with a multi-channel pulse-height analyzer. When two different characteristic X-rays almost simultaneously enter the detector, these X-rays cannot be recognized as separate pulses. Thus, an EDS spectrum exhibits a spectral peak at an energy position of the sum of energies of the two characteristic X-rays, together with spectral peaks due to characteristic X-rays of the specimen. The peak is called a "sum peak" and therefore, care must be taken for spectral analysis.
Related term escape peak
41. Gatan imaging filter
One of the post-column type energy filters produced by Gatan, which is installed behind the microscope column. This product consists of a sector analyzer of the magnetic-field type, quadrupole and octupole magnets for image enlargement, and a scintillator and CCD camera for image detection.
42. density of states
The number of electronic states per unit volume and per unit energy in energy band. The density of states (DOS) of the conduction band can be investigated by high energy resolution EELS. (The energy resolution is better than 1 eV for a TEM equipped with a thermal FEG and is about 0.3 eV for that with a cold FEG.) The DOS of the valence band is obtained by XES, which analyzes X-rays emitted from a specimen by the illumination of the incident electron beam. An energy resolution better than 1 eV is required for the analysis of the valence band. A recently-developed WDS analyzer provides an energy resolution of about 0.5 eV, giving us good DOS of the valence band.
Related term electronic structure, electron energy-loss spectroscopy, EELS, X-ray emission spectroscopy, XES
43. serial detection
In the acquisition of an energy-loss spectrum in EELS, the "serial detection" method uses a single channel detector (zero-dimensional detector) and measures the energy-loss spectrum in serial times along the energy axis. Recently, a detection method for EELS has changed to parallel detection.
Related term electron energy-loss spectroscopy, EELS, parallel detection
44. oscillator strength
"Oscillator strength" is a classical quantity corresponding to the probability of an electric dipole transition when an electron in the valence band or in the inner shell is excited to the conduction band. In the classical model, this transition can be considered as oscillations of bound electrons (harmonic oscillator) using the Lorentz model. The oscillator strength is a number of the oscillators, and corresponds to the quantum-mechanical probability of the electric dipole transition.
Related term Lorentz model
45. standardless quantitative analysis
A system by which quantitative analysis of target elements in EDS can be conducted without using the standard specimen in each analysis. In a recent TEM, many k-factors of the Cliff-Lorimer method, which have been measured separately, are stored and tabulated in the memory of the computer on the TEM. Quantitative analysis of the elements can be performed by referring the table without measuring the standard specimen. The system or method is called "standardless quantitative analysis." In TEM, when a specimen thickness is 10 nm or less, the Cliff-Lorimer method (thin film approximation) is generally used because the absorption effect and the fluorescence excitation effect can be neglected. For example, when a specimen is composed of two elements A and B, the mass concentration ratio of element A to element B is well assumed to be proportional to the measured characteristic X-ray intensity ratio of element A to element B (proportionality factor: k). The k factor, which depends on ionization cross section, fluorescent yield, X-ray absorption by material of detector window, etc, is actually obtained from characteristic X-ray measurements of a standard specimen. It is noted that the k factor is not a constant when a specimen is thick.
Related term energy-dispersive X-ray spectroscopy, EDS, k factor, Cliff-Lorimer method
46. spin orbit coupling
The magnitude of "spin orbit coupling" is different depending on whether the electron spin is parallel or anti-parallel to the orbital shell spin l, thus the two states possess different energies. In 2p electrons, L2 (total angular momentum j = l - s = 1/2) and L3 (total angular momentum j = l + s = 3/2) are formed. In an EELS spectrum of transition from the 2p occupied state to the 3d unoccupied state (band), L3 and L2 peaks sequentially appear in the energy-loss order of low to high. It should be noted that those peaks provide information on the energy splitting of the occupied state (several eV to 20 eV) but does not provide information on the unoccupied state expected by EELS. However, the intensity ratio of L3 to L2 varies from 2:1 due to the effect of the expected chemical-bonding state in the unoccupied state. When the experimental intensity profile is compared with theoretical calculations, information on the valence of 3d electrons is obtained, where the calculations must take account of the effect of core-hole interaction, correlation between 3d electrons and valences. The intensity ratio of L3 to L2 tends to be large at the high spin state whereas small at the low spin state.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES
47. three-window method
A method used for quantitative element (elemental) mapping by EELS. The process of the "three-window method" is as follows. Two background intensities before the inner-shell excitation of a certain element are acquired. The background intensity at the inner-shell excitation of the element is obtained by extrapolation using the two background intensities. Then, the obtained background intensity at the inner-shell excitation is subtracted from the spectral intensity of the inner-shell excitation. This method enables us to perform quantitative element mapping.
Related term electron energy-loss spectroscopy, EELS, two-window method
48. bremsstrahlung
"Bremsstrahlung" is an electromagnetic-wave radiation that is produced when an electron is rapidly decelerated by the Coulomb field of an atomic nucleus at a collision event of the electron with the atomic nucleus. Bremsstrahlung forms the background in an EDS spectrum.
Related term continuous X-ray
49. sector analyzer
One of the post-column type energy filters, which is installed behind the column of a TEM. Since the magnet of the analyzer is sector-shaped, this analyzer is called "sector analyzer." Its energy dispersion is 4 to 5 μm/eV for a 200 kV electron beam.
Related term energy analyzer
50. zero-loss peak
A sharp peak with an energy loss of 0 (zero) appearing in an EELS spectrum. The "zero-loss peak" is composed of no-scattered electrons and elastically scattered electrons. In a real spectrum, the peak shows an energy broadening (less than 0.7 eV) due to the energy spread of the incident beam. In the analysis of low-loss EELS spectra to obtain a dielectric function, it is important to precisely subtract the tail of the zero-loss peak.
Related term electron energy-loss spectroscopy, EELS
51. selection rule
In EELS, when interband transitions due to Coulomb interactions are considered, if only small-angle scattering is taken into account (small-angle scattering approximation), interband transitions are limited only to the dipole transition (dipole approximation). That is, only the transitions with the change of an angular orbital momentum being ⊿l = ±1 is allowed. The rule which clarifies the allowed and forbidden transitions is called "selection rule." Thus, the transitions from the 1s shell to the unoccupied p states (2p, 3p, etc.) occur. In ELNES, partial density of unoccupied states, instead of total density of states, is obtained. In the low energy-loss region corresponding to valence-electron excitations, scattering at large angles is allowed and this can give rise to transitions in which the selection rule does not hold.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES, valence-electron excitation
52. line analysis
In spectroscopic analysis, "line analysis" is to acquire a spectrum from a line scan of an electron beam on a specimen.
Related term point analysis, area analysis
53. occupied state
53-1. occupied state
In the occupied state in a molecule or crystal, the certain energy level and band are occupied by (valence) electrons. These electrons cannot move freely.
Related term unoccupied state
53-2. unoccupied state
In the unoccupied state in a molecule or crystal, the certain energy level and band are not occupied by (valence) electrons.
Related term occupied state
54. loss function
"Loss function" is given by the negative of the imaginary part of inverse of the dielectric function or Im[‐1/ε(ω)], where ε(ω) is the dielectric function. By removing the effect of multiple scatterings from an EELS spectrum, the EELS spectrum only due to single scattering is obtained. Next, the effect of the incident beam intensity and that of the aperture size from the spectrum are removed. And then, when the intensity of the obtained spectrum is normalized, the absolute value of the electron energy loss or the loss function is obtained. The loss function is the most important quantity obtained from EELS. By using Kramers-Kronig equation, the real part corresponding to the loss function is derived. From the real and imaginary parts, the dielectric function of the specimen is calculated. Various physical properties such as refractive index, optical reflectivity, optical absorption intensity etc. can be calculated from the dielectric function.
Related term electron energy-loss spectroscopy, EELS, dielectric function, Kramers-Kronig relation
55. onset energy
The onset energy means the energy at which a spectral intensity rises sharply in the EELS valence-loss spectrum or core-loss spectrum. This energy corresponds to the band gap in the valence-loss spectrum and to the bottom of the conduction band in the core-loss spectrum.
Related term core-loss spectrum, valence-loss spectrum (low-loss spectrum)
56. two-window method
A method used for qualitative element (elemental) mapping by EELS. In the "two-window method," a background intensity immediately before the inner-shell excitation (I1) and the peak intensity at the inner-shell excitation (I2) of a certain element are acquired. The ratio I = I2/ I1 is calculated. This method enables us to easily perform qualitative element mapping though it does not have a sufficient quantitative accuracy.
Related term electron energy-loss spectroscopy, EELS, three-window method
57. quantitative compositional analysis
"Quantitative compositional analysis" is to analyze not only the composition (kinds of constituent elements) but also the element abundance ratio (concentrations) of a substance. The composition is analyzed from the energy-peak positions in a spectrum and the element abundance ratio is quantitatively analyzed from the peak intensities.
Related term energy-dispersive X-ray spectroscopy, EDS, wavelength-dispersive X-ray spectroscopy, WDS, electron energy-loss spectroscopy, EELS
58. electron energy-loss spectroscopy (EELS)
58-1.electron energy-loss spectroscopy
When incident electrons strike constituent atoms in a specimen, some electrons are scattered while losing part of their energies (traveling speed becomes slower) through interactions with electrons and crystal lattices in the specimen. These electrons are called inelastically scattered electrons. A spectroscopy method, which obtains the energy spectra of the inelastically scattered electrons to perform qualitative and quantitative analysis of elements and electronic structure analysis from micro- or nano-areas, is called "electron energy-loss spectroscopy (EELS)." Inelastic scatterings analyzed by EELS are classified into three categories: (1) inner-shell electron excitations or core excitations: (50 to 2000 eV), (2) interband transitions due to valence-electron excitations (0 to 10 eV), and (3) plasmon excitations due to collective oscillations of free electrons (10 to 50 eV).
Related term core-loss spectrum, energy-loss near-edge structure, ELNES, extended energy-loss fine structure, EXELFS, chemical shift, density of states, valence-loss spectrum (low-loss spectrum), interband transition, plasma oscillation
58-2.parallel electron energy-loss spectroscopy
Parallel electron energy-loss spectroscopy (PEELS) is an EELS method which uses a parallel detector on the energy dispersive plane. PEELS has higher detection efficiency than the serial detection method that obtains a spectrum by changing the energy in serial times.
Related term electron energy-loss spectroscopy, EELS
59. electronic structure
59-1. electronic structure
"Electronic structure" means the electronic states in atoms, molecules and materials. In a solid, the orbits of outer-shell electrons of the atoms overlap each other, creating the valence band and the conduction band. The electronic structure is illustrated by the energy of the electron as a function of momentum and by the density distribution of the energy states in the bands.
Related term density of states
59-2. electronic structure analysis
Analysis for revealing electronic structures (energy and momentum) in a substance. Bonding features between atoms are analyzed.
Related term electron energy-loss spectroscopy, EELS, X-ray emission spectroscopy, XES, wavelength-dispersive X-ray spectroscopy, WDS
60. electron prism
60-1. electron prism
An "electron prism" is a (spectroscopic) analyzer to disperse energies of electrons similar to that a glass prism disperses the differences in wavelengths of light. The electron prism is essential for EELS analyzers and is effectively used for energy filtering of TEM images and diffraction patterns. They include the Wien filter, the omega filter, the alfa filter and the Castaing-Henry filter.
Related term Wien filter, omega filter, alfa filter, Castaing-Henry filter
60-2. electron biprism
An "electron biprism" is an electron-wave interferometer to obtain an electron hologram in the first step of electron holography. The electron biprism consists of a fine string electrode placed at the center of the incident electron beam (perpendicular to the electron beam) and parallel-plate ground electrodes placed at the both sides of the string electrode (parallel to the electron beam). The biprism is located below the objective lens in a TEM. A positive voltage is applied to the string electrode, so that the scattered wave transmitted through the object (object wave) passes through one side of the electrode whereas the wave directly coming from the electron source passes through the other side of the electrode. These two waves are attracted each other by the positive potential, and are superposed to form interference fringes (hologram). The interference fringes contain information on the change of the amplitude and phase of the object wave.
Related term electron holography, electron hologram
61. electron-probe microanalyzer (EPMA)
An instrument that performs element identification, quantitative element analysis and element distribution analysis in a specimen by illuminating a specimen surface with a fine electron probe and measuring characteristic X-rays generated. Since the "electron-probe microanalyzer (EPMA)" usually has an electron optical system similar to a SEM, backscattered electron images and secondary electron images are used for searching a specimen position to be analyzed. An optical microscope is also installed in an EPMA for searching a specimen position. Multiple wave-dispersive spectrometers (WDS) are incorporated in the EPMA for spectroscopic analysis of characteristic X-rays to achieve a high detection efficiency. The energy resolution of WDS is about 10 eV, whereas that of an energy dispersive spectrometer (EDS) is about 130 eV. The EPMA with the WDS is possible to conduct electronic structure analysis for favorable cases. The EPMA has a high performance of a detection limit of several tens of ppm and an error of 1% for quantitative composition analysis. The minimum specimen area to be analyzed is about 1 μm in diameter. A recent instrument equipped with an FE gun enables composition analysis of a specimen area of 0.1 μm in diameter.
Related term wavelength-dispersive X-ray spectroscopy, WDS, energy-dispersive X-ray spectroscopy, EDS
62. point analysis
In spectroscopic analysis, "point analysis" is to acquire a spectrum from a point on a specimen by stopping an electron beam at the point.
Related term line analysis, area analysis
63. Drude model
A model that treats oscillations of free electrons in a solid when an electric field is applied from outside. The "Drude model" allows plasma oscillations to be derived.
Related term plasma oscillation, plasmon, electron energy-loss spectroscopy, EELS, Lorentz model
64. characteristic X-ray
When one of inner-shell electrons is excited, an electron vacancy is formed in the inner-shell states. Then, an outer-shell electron, whose energy level is higher than that of the inner-shell electron, falls on the vacancy while emitting X-rays. The energy of the emitted X-rays corresponds to the energy difference between the outer-shell electron and the inner-shell electron. The emitted X-rays are called "characteristic X-ray(s)" and are characteristic of individual atoms. The characteristic X-rays are utilized for qualitative and quantitative element analysis on a micro-area.
Related term energy-dispersive X-ray spectroscopy, EDS, wavelength-dispersive X-ray spectroscopy, WDS, continuous X-ray
65. take-off angle
In the case of EDS analysis, "take-off angle" means that the angle at which characteristic X-rays emitted from the specimen are received with a detector placed above the specimen. This angle is defined by the angle of the line connecting the specimen center and the center of the detector against the normal plane to the optical axis. By setting this angle larger, signals cut by the specimen and specimen holder are reduced and the diffusion distance of the emitted X-rays in the specimen can be made shorter, thus the accuracy of quantitative analysis is improved. Previously, the take-off angle was set at 60°to 70°to reduce the above effects, which is called "High-Angle EDS" or "Top Take-off method." Recently, the take-off angle is however set at a low angle of ~20°by attaching the detector at the side of the objective polepiece because the detector is requested to place as near as possible to the specimen to increase the detection efficiency of the emitted X-rays or to increase the solid angle of the detector against the specimen, and the bore of the polepiece is requested to make small to obtain a high spatial resolution. This way of signal acquisition is called "Side Take-off method."
66. inner-shell (core) excitation
A process where an inner-shell electron is excited to the conduction band by absorption of X-rays or by collision with a high-energy electron or ion, and then an electron vacancy is created in the inner shell. EELS enables us to perform detailed analysis of inner-shell excitation.
Related term core-loss spectrum
67. core-loss spectrum
The high energy region (more than about 50 eV) of an EELS spectrum is called "core-loss spectrum." This spectrum, which is produced by exciting inner-shell electrons to the conduction band, enables us to perform qualitative and quantitative analysis of constituent elements, and to obtain the density of states of the conduction band of a material. The spectrum shows a structure specific to the material.
Related term electron energy-loss spectroscopy, EELS, chemical shift, energy-loss near-edge structure, ELNES, extended energy-loss fine structure, EXELFS, electronic structure analysis
68. nonisochromaticity
When the incidence direction of an electron beam to an energy filter is tilted from the optical axis of the filter, the energy spectrum undergoes an energy shift ⊿E = ⊿E(α) (α is the incidence angle with respect to the optical axis) due to the second-order aberration of the filter. This energy shift is termed "nonisochromaticity." When a finite-size energy slit is inserted, the energy range selected by the filter changes with the angular position of the slit.
69. wavelength-dispersive X-ray spectroscopy (WDS)
Wavelength-dispersive X-ray spectroscopy (WDS) is an element analysis method. Characteristic X-rays generated from a specimen are measured using Bragg reflections of X-rays with analyzing crystals, based on the diffraction angles of the reflected X-rays caused by the analyzing crystals. The analyzing power of light elements surpasses EDS. That is, WDS can analyze elements from boron (B) on down. However, the detection efficiency of WDS is lower than EDS. Thus, the illumination current of the electron beam for WDS needs to be set larger than that for EDS (several nA to several 100 nA). As a result, care must be taken for the beam damage to the specimen to suppress the damage. Normally, the resolution of WDS is about 10 eV. Its quantification accuracy is 0.1 to 0.2%. Recently, a high energy-resolution (exceeding 1 eV) analyzer that uses a grating has been developed. Due to its superbly high resolution, this analyzer can be used for analyzing the density of states of the valence band. "WDX" is also used as the abbreviation of wavelength-dispersive X-ray spectroscopy.
Related term characteristic X-ray, energy-dispersive X-ray spectroscopy, EDS
70. luminous efficiency
The ratio of the emission energy to the absorbed energy when a fluorescent substance is excited by an incoming radiation and then fluorescence emission is generated.
71. parallel detection
In the acquisition of an energy-loss spectrum in EELS, the "parallel detection" method uses a one-dimensional detector or a two-dimensional detector to efficiently measure the energy-loss spectrum in parallel.
Related term EELS, serial detection
72. interband transition
72-1. interband transition
When the Bragg reflection is treated by the dynamical theory, dispersion surfaces (equi-energy surface), which give allowable wave numbers (equivalent energy planes) near Bragg reflections, are produced. Two dispersion surfaces are produced for each reflection. When the incidence direction of the electron beam onto a specimen crystal (the excitation error of the Bragg reflection) and the surface normal of the specimen are given, the allowed points on the dispersion surfaces are determined. As a result, the wave numbers and amplitudes of the allowed waves in the crystal are determined. If the crystal is perfect, these quantities are kept unchanged. If a defect, for example a stacking fault exists in the crystal, redistribution of the amplitudes occurs on the dispersion surfaces. Wave transfer to a different dispersion surface is called "interband transition." On the other hand, wave transfer within the same dispersion surface is called "intraband transition." The above interband transition in the case of a stacking fault takes place in the scope of elastic scattering. In the case of inelastic scattering, we can consider similar dispersion surfaces which are a little different in their energy from those of elastic scattering. Interband transition occurs at small-angle scattering of thermal diffuse scattering, but intraband transition occurs for plasmon scattering. In the case of core excitation, if interaction that gives rise to the core excitation is small (normally, small), intraband transition occurs. The electrons suffered by the intraband transition produce a similar image to the original image (without transition). However, since the symmetry of the Bloch wave of a dispersion surface is different from that of the other surface, the electrons suffered by interband transition do not form a similar image to the original image. (This term is different from "interband transition" used in solid state physics. In the case of the interband transition in solid state physics, the energy of the electron changes but it does not change in electron diffraction.)
Related term interband transition, dynamical diffraction, dispersion surface, inelastically scattered electron
72-2. interband transition
A phenomenon in which electrons in a crystal make transitions from the valence band to the conduction band. EELS enables us to obtain the band gap energy from the onset energy of a spectrum that reflects interband transitions.
Related term valence-loss spectrum (low-loss spectrum)
73. band gap
The band gap means energy width of the forbidden band, which is situated between the valence band and the conduction band and does not allow electrons in a crystal to exist. The larger the band gap, the greater the insulation quality is.
Related term valence-loss spectrum (low-loss spectrum)
74. peak-to-background ratio
The "peak-to-background ratio" is defined as the ratio of the peak intensity to the background intensity in a spectrum. (1) In an ELNES spectrum of EELS, the ratio is low because of its high-background signal. As the accelerating voltage of the incident electron beam is increased, the peak-to-background ratio is improved, because multiple scattering is decreased and the effective acceptance angle is increased. (2) In an EDS spectrum measured in a TEM, the ratio of the characteristic X-ray peak intensity to the background intensity is very high. When the accelerating voltage is increased, the ratio is further improved because the probability of bremsstrahlung (background) is decreased.
Related term bremsstrahlung, continuous X-ray
75. optical absorption spectrum
An optical absorption spectrum is acquired by the absorption of specific (wavelength) light in a substance, when the substance is illuminated with visible light.
Related term X-ray absorption spectroscopy, XAS, electromagnetic wave
76. unoccupied state
In the unoccupied state in a molecule or crystal, the certain energy level and band are not occupied by (valence) electrons.
Related term occupied state
77. finger printing
"Finger printing" is used for ELNES analysis in EELS. In this technique, the spectra of known chemical bonding states are prepared as fingerprints in advance. Then, an unknown spectrum is identified to a certain bonding state by comparing with the known spectra.
Related term electron energy-loss spectroscopy, EELS, energy-loss near-edge structure, ELNES
78. Fermi level
"Fermi level" is the highest energy level occupied by electrons in the ground state of a crystal. That is, at the ground state, no electrons occupy the levels above the Fermi level.
79. depth profile
"Depth profile" means a result of compositional analysis along the depth direction in a specimen, which is obtained by milling a specimen step by step from the surface and by analyzing the milled surface.
80. impurity level
"Impurity level" is defined as an energy level created in the band gap in a semiconductor, which is introduced by impurity atoms.
Related term cathodoluminescence
81. plasma oscillation
Plasma oscillations generally mean various oscillations of electrons and ions in a plasma state. The plasma oscillations detected and analyzed by EELS are collective oscillations of free electrons (volume plasmon) and oscillation modes of electrons on a solid surface (surface plasmon). The oscillation frequency of the volume plasmon is proportional to the square root of the electron density. The oscillation frequency of the surface plasmon is (1/√2) times that of the volume plasmon when the solid surface is exposed to vacuum.
Related term plasmon, valence-loss spectrum (low-loss spectrum)
82. plasmon
"Plasmon" expresses the quantum form of plasma oscillation. Since the plasmon (volume plasmon) is longitudinal wave oscillation, it cannot be observed by the optical method but can be directly observed as a plasmon excitation spectrum by EELS.
Related term valence-loss spectrum (low-loss spectrum)
83. analytical electron microscopy (AEM)
A microscopy method that adds analytical functions, such as EDS and EELS to a TEM, in order to perform qualitative and quantitative analysis of elements and/or electronic structure analysis from micro- or nano-areas subjected to TEM observation.
Related term energy-dispersive X-ray spectroscopy, EDS, electron energy-loss spectroscopy, EELS
84. analysis region
The size of the "analysis region" (spatial resolution) in EDS analysis is determined by not only the diameter of the incident electron beam but also the beam broadening within a specimen that depends on accelerating voltage, specimen thickness and constituent elements. For an accelerating voltage of 200 kV, the analysis region is 10 to 50 nm in diameter. The beam broadening decreases with the increase of the accelerating voltage.
85. Bethe Ridge
"Bethe Ridge" is a tail-like peak (ridge) which appears in the expression of energy loss against scattering angle, E(θ) for the collision between incident electrons and quasi-free electrons in a solid. Taking account of a similar phenomenon in the case of X-ray scattering, Bethe Ridge is also called "Compton peak." In a treatment of classical dynamics, the position of Bethe Ridge is expressed as E/E0~sin2θ,where E0 is the energy of incident electrons. Bethe Ridge is observed by taking angle-resolved EELS spectra.
86. post-column type
An energy filter or an energy analyzer that is installed behind the column of a TEM is classified as "post-column type." This type includes a GIF and a Tridiem.
Related term GIF, Tridiem, in-column type
87. Moellenstedt analyzer
One of the in-column type energy filters, which is installed between the intermediate and projector lenses in a TEM. In the "Moellenstedt analyzer," lens aberration is used to get energy dispersion, instead of deflection by a magnetic field or an electric field. The initial Moellenstedt analyzer used an electrostatic lens, but the modern Moellenstedt analyzer adopts a magnetic field lens for a 100 kV or higher voltage TEM.
Related term energy analyzer
88. area analysis
In spectroscopic analysis, "area analysis" is to acquire a spectrum from an area (two-dimensional) scan of an electron beam on a specimen.
Related term line analysis, point analysis
89. monochromator
A device that can monochromate an electron beam. In a 200 kV TEM, the energy spread of the electron beam is about 2 eV for an LaB6 thermionic-emission electron gun, about 0.7 eV for a Schottky type electron gun and about 0.4 eV for a cold type field-emission electron gun. The use of the "monochromator" dramatically decreases the energy spread, down to less than 0.1 eV. Thus, the monochromator improves the energy resolution of EELS. The use of a monochromator in EELS is crucial for investigation of the electronic structures of solids.
Related term Wien filter, energy analyzer
90. dielectric function
A function that expresses the dielectric constant of a substance as a function of frequency. In general, the "dielectric function" is expressed to be a complex quantity as a function of frequency and wave number vector. From the dielectric function, optical properties of a substance (such as refractive index and reflectivity as a function of frequency) are derived. From an EELS spectrum, the loss function is obtained which is proportional to the imaginary part of the reciprocal of the complex dielectric function.
Related term valence-loss spectrum (low-loss spectrum), electron energy-loss spectroscopy, EELS
91. retarding
"Retarding" means the retardation of an electron beam in a TEM. In the optical system of the electron microscope, a certain voltage is applied to an additional electrode, a lens or a specimen stage to lower (retard) the velocity of the electron beam. Retarding of the electron beam improves the energy resolution of EELS spectra. As an attempt for multi-functionality of TEM, a retarding voltage is applied to the specimen stage for the observation of a low-accelerating-voltage TEM image while the accelerating voltage of the TEM itself is kept unchanged. In addition, changing the retarding potential (voltage) enables us to measure the energy distribution of backscattered electrons and to take a reflection diffraction pattern only from elastically scattered electrons.
Related term electron energy-loss spectroscopy, EELS
92. continuous X-ray
"Continuous X-rays" are X-rays having continuous wavelength distribution, which are produced when electrons are rapidly decelerated by the Coulomb field of an atomic nucleus. An X-ray tube used in a laboratory utilizes this phenomenon (Bremsstrahlung).
Related term bremsstrahlung
93. Rowland circle
A circle, which is virtually drawn tangent to the center point of a spherical concave grating with a diameter equal to the radius of curvature of the grating. (This circle is half the size of the circle produced by the concave grating.) When a slit is placed at an arbitrary point on the "Rowland circle" and is illuminated with light. The light whose source is at the slit causes diffraction with the concave grating and forms an aberration-free spectrum on a position of the Rowland circle.
Related term concave grating, wavelength-dispersive X-ray spectroscopy, WDS
94. Lorentz model
A model that considers oscillations of bound electrons in a solid when an electric field is applied from outside. The "Lorentz model" gives the classical model of the valence and inner-shell electron excitations.
Related term valence-loss spectrum (low-loss spectrum), electron energy-loss spectroscopy, EELS, oscillator strength, Drude model
95. live time
95-1. live time
The live time is an effective measurement time. That is, the live time equals to the net measurement time determined by subtracting the dead time from the total measurement time. This concept is used for EDS.
Related term energy-dispersive X-ray spectroscopy, dead time, live time scan
95-2. live time scan
Live time scan is an EDS measurement technique to scan an electron probe over an area by varying the dwell time of the probe so that the effective measurement time (live time) for characteristic X-rays becomes equal at each scanning point. If the dwell time is set to a definite time for all the scanning points, the dead time (rate) for the scanning points with a large X-ray generation increases. Thus, the generated X-rays are counted smaller than the true counts. As a result, the concentration of the element concerned is underestimated. To avoid this phenomenon, the dwell time at each scanning point is varied so as to make the live time equal for all the points (live time scan). Therefore, the live time scan enables us to acquire the correct two-dimensional element map of the specimen.
Related term energy-dispersive X-ray spectroscopy, dead time, characteristic X-ray, area analysis, live time
96. process time
The process time is a time index to select whether to use the EDS analysis focusing on its energy resolution or focusing on its analysis time (throughput). By changing the process time, an EDS analysis suitable for analysis purpose can be carried out. Some EDS manufacturers name it as "time constant." In the element analysis by EDS, characteristic X-rays generated from a specimen are subjected to dispersion according to their energy. In the process of EDS, averaging of the noise components of X-ray signals which are detected with a semiconductor detector is performed to obtain accurate energy values of the X-rays. By changing the process time, the averaging time is changed. If the process time (averaging time) is longer, a high-energy resolution EDS spectrum is acquired because the measurement error of the X-ray energy values decreases. However, the X-rays entering the detector in the averaging time cannot be measured, and then the dead time increases. If the process time (averaging time) is shorter, the dead time decreases and the time required for analysis is shortened. However, since the error of the X-ray energy values increases because of a shorter averaging time, the energy resolution is sacrificed.
Related term energy-dispersive X-ray spectroscopy, dead time, energy resolution
97. volume plasmon
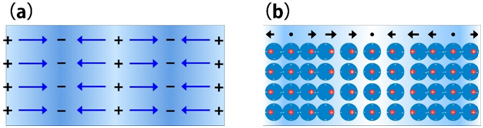
Collective oscillations of free electrons with a longitudinal wave or compressional-wave, which are induced in metals by an electron beam or a charged-particle, are called "volume plasmon". The volume plasmon is excited also in semiconductors and insulators. Its oscillation energy (plasmon energy) is proportional to the square root of the free electron density (valence electron density for semiconductors and insulators). The volume plasmon is directly observed as a peak in an electron energy-loss spectrum. It should be noted that the volume plasmon cannot be excited nor can be observed with a light wave or a transverse wave. When a high-speed electron beam is incident on a solid metal, the Coulomb force formed by the beam induces a density change (compression) in the homogeneously distributed free electrons in the metal. With the induced Coulomb force as a driving force, a longitudinal oscillation wave of the free electrons with a specific frequency is created (see Fig.1). Quantization of this collective motion of the free electrons is called volume plasmon. The energy of the volume plasmon, EP, is expressed by the following equation.
Here, ωp is the angular frequency of the plasma oscillation. ħ= h/2π is Planck’s constant. e, m, and N respectively express the elementary charge of an electron, the mass of an electron, and the density of the free electrons. The plasmon energy is proportional to the square root of the free electron density in a metal. Although the volume plasmon is originally considered for free electrons, it is excited also in semiconductors and insulators as a collective oscillation of the whole valence electrons. The valence electrons vibrate collectively against positive ion cores. The energy or frequency of the plasmon is calculated by substituting the density of the valence electrons into the above equation.
Fig. 1(a) Scematic of plasmon induced by free electrons. Metal is electrically nutral because free electrons and positive ions consisting of an atomic nuclei and inner shell electrons are homogenously distributed. When a high energy electron beam is incident on a metal from outside, the Coulomb force of the incident beam creates a density change in the free electrons, or positive and negative charged regions (indicated by (+) and (-) in Fig. 1(a)). With the electric field (indicated by blue arrows) caused by the density change as a driving force, the free electrons collectively starts a characteristic oscillation (plasma oscillation). (b) Schematic of plasmons of bounded electrons. Red dots and blue circles respectively show positive ions and valence electrons. The bounded valence electrons are displaced by the Coulomb force of the incident electron beam, density changes of the valence electrons being created. Then, the valence electrons collectively oscillate with plasma frequency. Black arrows at the top of Fig. 1(b) represent polarization caused by the displacements of the valence electrons. Plasma vibration of the bounded electrons is a longitudinal wave vibration caused by polarization of the atoms. The charge density of Al is N = 1.8 × 1023 e/cm3. The plasmon energy is calculated to be 15.7 eV, showing a good agreement with an experimental value of 15.0 eV. For monovalent metals (Li, Na, etc.), the calculated energies agree well with the energies experimentally obtained. For diamond (insulator), the plasmon energy is calculated to be 31 eV using the valence electron density (N = 7.0 × 1023 e/cm3), showing a rather good agreement with an experimental energy of 34 eV. In the cases of ionic crystals such as LiF, NaCl etc., their plasmon energies calculated using the valence electron densities well reproduce the energies experimentally obtained. Table 1 shows the plasmon energies for various materials. However, for a material in which interband transitions strongly occur close to the expected plasmon energy, the experimental plasmon energy can be largely deviated from the expected energy. For example, in the case of Ag, the plasmon energy expected from the valence electron density of N=0.59×1023 e/cm3 is 9.0 eV but the experimental plasmon energy is observed at 3.9 eV due to a strong interband transition of the d orbital electrons at 4.0 eV.