结晶等(结晶构造、材料样品)
目录
- 1 1. Anisotropy
- 2 2. glide plane
- 3 3. inclusion
- 4 4. rotation axis
- 5 5. rotary-inversion axis
- 6 6. interface
- 7 7. chemical order/disorder
- 8 8. ordered and disordered structure
- 9 9. boundary
- 10 10. mirror plane
- 11 11. ferromagnetic material
- 12 12. ferroelectric material
- 13 13. chirality
- 14 14. intermetallic compound
- 15 15. crystallographic space group
- 16 16. crystal orientation
- 17 17. crystal structure
- 18 18. crystal growth
- 19 19. space lattice
- 20 20. atomic plane
- 21 21. lattice defect
- 22 22. lattice parameter (constant)
- 23 23. lattice plane
- 24 24. solid solution
- 25 25. GP zone
- 26 26. magnetic domain
- 27 27. antiferromagnetic material
- 28 28. real space
- 29 29. real lattice
- 30 30. quasicrystal
- 31 31. zone axis
- 32 32. paraelectric material
- 33 33. precipitate
- 34 34. stacking fault
- 35 35. twins
- 36 36. phase transformation
- 37 37. residence time
- 38 38.Polycrystal
- 39 39. single crystal
- 40 40. unit cell
- 41 41. short-range order parameter
- 42 42. Substitution
- 43 43. centro-symmetry
- 44 44. long-range order parameter
- 45 45. superlattice
- 46 46. long period structure
- 47 47. dislocation
- 48 48. crystallographic point group
- 49 49. point defect
- 50 50. Isotropy
- 51 51. Neel wall
- 52 52. inversion center
- 53 53. inversion domain boundary
- 54 54. amorphous
- 55 55. composite crystal
- 56 56. Impurity
- 57 57. incommensurate structure
- 58 58. Bloch wall
- 59 59. ferroelectric domain
- 60 60. displacement
- 61 61. deformation
- 62 62. segregation
- 63 63. modulated crystal
- 64 64. Miller index
- 65 65. dielectric material
- 66 66. dielectric constant
- 67 67. screw axis
1. Anisotropy
"Anisotropy" means that physical properties of a crystalline substance are different depending on its crystal orientations.
Related term isotropy
2. glide plane
A symmetry element of the crystallographic space group. When a crystal is mirrored with respect to a certain crystal plane (mirror plane) and successively translated parallel to this mirror plane by a length of 1/2 or 1/4 of the crystal unit-cell, the translated crystal can be equivalent to the original crystal. In this case, this plane is called "glide plane." The glide planes are denoted by g, instead of m in the case of mirror planes.
Related term crystallographic space group, screw axis
3. inclusion
Inclusions are impurity particles captured in a solid.
4. rotation axis
A symmetry element of the crystallographic point group. When the whole crystal (constituent atoms) is rotated by a certain angle (180°, 120°, 90° or 60°) with respect to a line, the rotated crystal can be equivalent to the crystal before rotation. In this case, this line is called "rotation axis." Corresponding to the respective rotation angles, there exist 2-fold, 3-fold, 4-fold and 6-fold rotation axes.
Related term crystallographic point group, rotary-inversion axis, mirror plane, inversion center
5. rotary-inversion axis
A symmetry element of the crystallographic point group. When the whole crystal (constituent atoms) is rotated by an angle of 90° with respect to a line, and successively an inversion is operated for the rotated crystal, the resultant crystal can be equivalent to the crystal before the operations. In this case, the combination of the two symmetries is called "rotary-inversion axis." Only the 4-fold rotary-inversion axis is a symmetry of the crystallographic point group.
Related term crystallographic point group, rotation axis, mirror plane, inversion center
6. interface
6-1. Interface
An "interface" is a boundary surface between two different phases. In the case of crystalline phase, the interface often takes a specific crystalline plane. In materials science, it is important to analyze grain boundaries in metals, and interfaces (boundary surfaces) on multiple layers in semiconductors and ceramics.
Related term boundary
6-2. Graphical User Interface
"GUI" is a user interface that provides intuitive operations using a computer graphics and a pointing device. Since GUI offers user-friendly operation and high visualization, it is widely used as a major interface for commercial OS. In the TEM, GUI is used to facilitate instrument operation and display of image-data. It is, however, noted that GUI is not always effective because different operations cannot be carried out simultaneously.
7. chemical order/disorder
An order about the arrangement of constituent elements in a compound or an alloy. For example, in the case of a compound containing two atoms A and B, a "chemical order" implies that each atom is alternately arranged in an ordered fashion. When each atom is arranged in a random fashion, this state is called a "chemical disorder." The chemical disorder takes place when the chemical properties of the constituent atoms are similar to each other. Compounds or alloys can take a chemical disorder state at high temperature and undergo a chemical order state at low temperature.
8. ordered and disordered structure
For example, in the high-temperature phase of Cu3Au, Cu and Au randomly occupy the lattice points of the face-centered structure. In the low-temperature phase, Au occupies the position of origin and Cu occupies the three face-centered positions. In this manner, the "ordered and disordered structure" means that when the external condition (temperature) changes, the structure of a substance changes where the constituent atoms are arranged orderly or randomly. The order-disorder transformation occurs in not only alloys but also inorganic compounds. If an ordered structure is formed, diffraction spots specific to the structure appear.
Related term long-range order parameter, short-range order parameter
9. boundary
9-1. boundary
In the case of crystal, a "boundary" separates crystals with different orientations or phases, or it separates crystals with different compositions or structures. Boundaries include stacking fault, twin boundary, inversion domain boundary, anti-phase domain boundary, grain boundary, and multi-layer boundary.
Related term stacking fault, twins, inversion domain boundary, grain boundary
9-2. boundary condition
The boundary condition is the condition which should be satisfied by the solution function of the differential equation of a scattering problem at the boundary of a crystal. The boundary conditions in the case of the Bethe' s method for the amplitudes of transmitted and diffracted waves are as follows: 1) The amplitudes of the incident electron wave and those inside the crystal are the same at the entrance surface. 2) The tangential components of those electron waves are the same at the entrance surface.
Related term Bethe's method
9-3. grain boundary
"Grain boundary" separates respective crystalline particles with different orientations in a polycrystalline solid. Grain boundaries are also called crystalline grain boundaries. High-resolution HAADF-STEM image of polycrystalline silicon taken at an accelerating voltage of 300 kV. The boundary indicated by red allows is a grain boundary of crystalline silicon. The image reveals that the dumbbells of silicon atoms change their orientations across the boundary. This grain boundary is expressed by Σ3, [110]/{111}.
Related term boundary
9-4. inversion domain boundary
"Inversion domain boundary" separates two adjacent crystals which coincide with each other by an inversion operation.
Related term boundary
9-5. anti-phase boundary
"Anti-phase boundary" separates two adjacent crystals which have the same crystallographic orientation but have a 180°phase shift (a shift of half period) each other. Anti-phase boundaries frequently appear in the ordered phase of a binary alloy.
10. mirror plane
A symmetry element of the crystallographic point group. When the whole crystal (constituent atoms) is mirrored with respect to a certain plane, the mirrored crystal can be equivalent to the crystal before the mirror operation. In this case, this plane is called "mirror plane."
Related term crystallographic point group, rotation axis, glide plane, inversion center
11. ferromagnetic material
11-1.ferromagnetic material
A material that consists of atoms having magnetic moments, which align parallel to each other, thus exhibits strong spontaneous magnetic polarizations without any external magnetic field. In a magnetic domain, all the magnetic moments take the same direction. When an external magnetic field is applied to a ferromagnetic material, strong magnetic moments appear in the direction of the magnetic field, and residual magnetic moments exist even after the magnetic field is removed, thus exhibiting a hysteresis loop of the magnetic moment against the magnetic field. Certain materials show ferromagnetism below a phase transformation temperature, called the Curie temperature Tc, and are paramagnetic (disordering of the moments) above the temperature.
Related term paramagnetic material, diamagnetic material, antiferromagnetic material, magnetic domain
11-2.antiferromagnetic material
An "antiferromagnetic material" consists of atoms having magnetic moments but the neighbouring magnetic moments are aligned anti-parallel to each other, thus the net magnetic moment of the material is 0 (zero). The material is magnetized in the direction of a magnetic field like a paramagnetic material when placed in a weak magnetic field. When the magnetic field is increased, it is strongly magnetized in the direction of the magnetic field like a ferromagnetic material. It exhibits a double-hysteresis loop against changes of the magnetic field.
Related term ferromagnetic material, paramagnetic material, diamagnetic material
12. ferroelectric material
12-1. ferroelectric material
A material that has spontaneous electric polarizations, which can be reversed by the application of an external electric field. In a ferroelectric domain, all the polarizations take the same direction. When an external electric field is applied to a ferroelectric material, strong polarizations appear in the direction of the electric field, and residual electric polarizations exist even after the electric field is removed, thus exhibiting a hysteresis loop of polarization against the external field. Certain materials show ferroelectricity below a phase transformation temperature, called the Curie temperature Tc, and are paraelectric above this temperature.
Related term paraelectric material, ferroelectric domain, antiferroelectric material
12-2. antiferroelectric material
An "antiferroelectric material" indicates such a crystal that consists of two sublattices which have anti-parallel dielectric polarizations each other, thus the net polarization of the crystal being 0 (zero). The material is polarized in the direction of an electric field like a paraelectric material when placed in a weak electric field. When the electric field is increased, strong electric polarization is generated in the direction of the electric field like a ferroelectric material. It exhibits a double-hysteresis loop against changes of the electric field.
Related term ferromagnetic material, paraelectric material
13. chirality
As in the case of the human left-and-right hands, "chirality" means that two certain structures having mirror-image relation to each other cannot coincide through rotation operations. These structures are possible for crystals belonging to the point groups which do not have mirror symmetry and centro-symmetry.
14. intermetallic compound
A compound that consists of two or more metallic elements.
15. crystallographic space group
Since the unit cells are arranged repeatedly in a crystal, symmetry operations by which a point in a crystal is transformed to an equivalent point of a neighboring unit cell are allowed as symmetry operations leaving the crystal unchanged. These symmetry elements are screw axes and glide planes. "Crystallographic space groups" are constructed by the combinations of the screw axes and the glide planes with the symmetry elements of the crystallographic point groups. There exist 230 space groups.
Related term screw axis, glide plane, crystallographic point group, convergent-beam electron diffraction, CBED, forbidden reflection, extinction rule
16. crystal orientation
"Crystal orientation" is defined by the plane (Miller) indices of the lattice plane of a crystal. In observation of an electron microscope image using a TEM, the particular crystal orientation (usually, orientation expressed by the low-order indices) is aligned to the direction of the incident electron beam. The use of a Kikuchi pattern enables us to easily align the crystal orientation with high accuracy.
Related term Kikuchi pattern
17. crystal structure
17-1. crystal structure
"Crystal" is an object in which a unit composed of atoms and molecules is periodically arranged. The unit is called a unit cell. If we know the atomic arrangement in the unit cell, we obtain the entire knowledge of the crystal. The arrangement of atoms and molecules in the unit cell is called "crystal structure." In TEM, convergent-beam electron diffraction (CBED) is used for the detailed analysis of the crystal structure.
Related term unit cell, lattice parameter (constant), crystal structure analysis, convergent-beam electron diffraction, CBED
17-2. crystal structure analysis
For crystal structure analysis, the electron microscope (TEM) image is not used, but the electron diffraction is used because the spatial resolution of the TEM is around 0.1 nm, but the electron diffraction pattern achieves a spatial resolution of 0.001 nm. In structure analysis, there are two methods; one is to use kinematical diffraction, the other is to use dynamical diffraction. The former is applied when a crystalline specimen is thin and consists of light elements and the dynamical diffraction effect can be neglected. Actually, this method is used for protein crystal analysis. The intensities of each reflection are measured from a diffraction pattern. The phases of the reflections are obtained from the real and imaginary parts of the scattering factors which are obtained by Fourier transform of the corresponding TEM image. Then, the structure is obtained by Fourier synthesis of the intensities and phases. The latter method, which uses convergent-beam electron diffraction (CBED), is applied to structure analysis of nano-scale crystals in the field of materials science. The CBED method has more advantage to study the secondary structure of solid materials, or local structures due to lattice defects and lattice strain than to study the primary structure of crystals. A disk diffraction pattern is acquired by illumination of an electron beam with an incidence angle of several 10 mrad on a small specimen area of a diameter of 10 nm or less. The acquired disk diffraction pattern (CBED pattern) exhibits a two-dimensional rocking curve (intensity distribution) corresponding to the spread of the incidence beam angle. (The CBED pattern appears to be complex due to the dynamical diffraction effect, thus the pattern is greatly different from the Laue function or the rocking curve which is expected from kinematical diffraction.) The crystal structure is solved by the fitting between the simulated CBED pattern obtained by the full dynamical calculation and the experimentally-acquired CBED pattern. Since the phases of the diffracted waves are reflected in the diffraction intensities due to multiple diffraction effects, separate determination of the phases of the diffracted waves is not necessary, which is needed in the case of kinematical diffraction. In addition, an energy filter is effectively used to remove inelastic scattering. The third method is the pre-session method. In this method, to avoid the strong dynamical diffraction effect using illumination of a cone-shaped incident beam on a crystalline specimen with a tilt angle of several degrees from a zone axis, the intensities produced by the cone illumination are added for each reflection. The crystal structure is solved by applying the kinematical theory using the obtained intensities, where the direct method for X-ray diffraction is used to estimate the phases of the diffracted waves.
Related term crystal structure, electron diffraction, kinematical diffraction, dynamical diffraction, convergent-beam electron diffraction, CBED, rocking curve, Laue function, energy filter
17-3. crystal structure factor
The crystal structure factor gives the amplitude and phase of a diffracted wave from a crystal. The factor is determined by the atom species and their positions in a unit cell.
Related term atom form factor
17-4. crystal structure image
HREM, which allows wave interference between transmitted and diffracted waves to be caused, enables us to obtain an image exhibiting the crystal structure of a thin crystalline specimen (thickness <10 nm). This image is obtained at the defocus condition, which is determined by the spherical aberration of the objective lens and the accelerating voltage of the incident beam (Scherzer focus). The image is taken by setting the electron beam parallel to a low-order zone axis and by passing a transmitted beam and many diffracted beams through the objective aperture. When the image is not taken at Scherzer focus, it is called "lattice image," which does not always correspond to the crystal structure. Three important factors to take an image corresponding to the crystal structure are: (1) Preparation of a sufficiently thin crystalline specimen, (2) High-accuracy adjustment of the crystal orientation, and (3) Adjustment of the Scherzer focus condition.
Related term high-resolution electron microscopy, HREM, phase contrast, Sherzer focus, zone axis, lattice image
18. crystal growth
"Crystal growth" means that a single crystal or a polycrystal grows from a crystalline substance through various processes such as solidification from melt, solidification from vapor, and precipitation from solvent.
Related term single crystal, polycrystal
19. space lattice
"Space lattice" means a periodic array of a unit lattice (a parallelepiped) in a space. The space lattice is classified into 7 crystal systems according to lattice parameters (a, b, c, α, β, γ). Unit lattices of several crystal systems can locate within them. According to the location of the lattice points, face-centered lattice, body-centered lattice and base-centered lattice are created. As a result, it amounts to 14 type lattices, which are termed Bravais lattices. Lattices other than primitive lattice cause forbidden reflections to occur.
Related term unit cell, lattice parameter (constant), forbidden reflection, extinction rule
20. atomic plane
A crystal is regarded as a stacking of atomic planes, which is created by a regular array of atoms. In a (perfect) crystal, different kinds of atomic planes stack periodically.
Related term lattice plane, stacking fault
21. lattice defect
Disorder of the atomic arrangement in a crystal. In a perfect crystal, constituent atoms create a regular and an ordered arrangement. However in a real crystal, the order of arrangement is violated and structural disorder exists. "Lattice defect" is classified into planar faults, line defects and point defects, in terms of its form.
Related term stacking fault, dislocation, point defect
22. lattice parameter (constant)
The lattice parameters are the quantities specifying a unit cell or the unit of the periodicity of the atomic arrangement. The lattice parameters (constants) are composed of "a, b, c," lengths of the unit cell in three dimensions, and "α, β, γ," their mutual angles.
Related term unit cell, crystal structure
23. lattice plane
In a crystal, equivalent atomic planes are aligned in parallel with an equal distance. Thus, a crystal is regarded as an assembly of planes created by atoms. In this context, a set of parallel planes is named a "(crystal) lattice plane." The distance between the neighboring planes is termed the spacing of the plane. In a crystal, there are many lattice planes with different orientations.
Related term atomic plane
24. solid solution
When two or more kinds of substances are mixed and have a uniform structure (particularly in the case of ion crystals and semiconductors), the mixed crystal is called a solid solution. Different atoms are randomly arranged on equivalent lattice sites in the solid solution. Below a certain temperature, the different atoms can take an ordered arrangement.
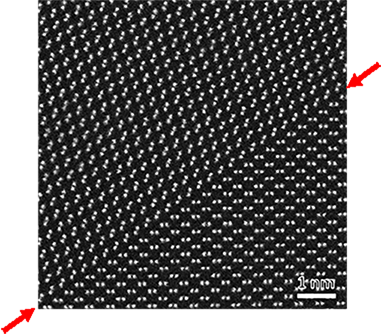
25. GP zone
"GP zone" means a plate that is composed of solute atoms segregated on a plane. The zone was discovered independently by A. Guinier and G. D. Preston through the analysis of streaks extending from X-ray Laue spots. It is named Guinier-Preston (GP) Zone after the two researchers. In the case of an age-hardened alloy that consists of Al or Mg but includes minute solute atoms (Cu, etc.), when its super-saturated solid solution is rapidly cooled and undergoes an aging treatment at a low temperature, solute atoms (Cu, etc.) are precipitated as one or two atomic planes with lattice matching on the {001} plane of the parent phase. It is noted that they do not appear as precipitates in the equilibrium state. The GP zone causes age hardening of alloys containing Al or Mg. In a bright-field image, the GP zone shows a line contrast due to lattice distortions. In a high-resolution image, the arrangement of solute atoms is directly observed.
Bright-field image of an AlCu alloy containing GP zones taken at an accelerating voltage of 100 kV. GP zones (Cu precipitates) are seen to be bright (white) lines, indicated by white allows, because they do not satisfy Bragg condition.
26. magnetic domain
A "magnetic domain" means a region where the directions of all the magnetizations of respective atoms are the same.
Related term ferromagnetic material
27. antiferromagnetic material
27-1. magnetic material
An "antiferromagnetic material" consists of atoms having magnetic moments but the neighbouring magnetic moments are aligned anti-parallel to each other, thus the net magnetic moment of the material is 0 (zero). The material is magnetized in the direction of a magnetic field like a paramagnetic material when placed in a weak magnetic field. When the magnetic field is increased, it is strongly magnetized in the direction of the magnetic field like a ferromagnetic material. It exhibits a double-hysteresis loop against changes of the magnetic field.
Related term ferromagnetic material, paramagnetic material, diamagnetic material
27-2. diamagnetic material
A material that is magnetized in the reverse direction against a magnetic-field direction when placed in the magnetic field, and demagnetized when the magnetic field is removed.
Related term ferromagnetic material, paramagnetic material, antiferromagnetic material
27-3. ferromagnetic material
A material that consists of atoms having magnetic moments, which align parallel to each other, thus exhibits strong spontaneous magnetic polarizations without any external magnetic field. In a magnetic domain, all the magnetic moments take the same direction. When an external magnetic field is applied to a ferromagnetic material, strong magnetic moments appear in the direction of the magnetic field, and residual magnetic moments exist even after the magnetic field is removed, thus exhibiting a hysteresis loop of the magnetic moment against the magnetic field. Certain materials show ferromagnetism below a phase transformation temperature, called the Curie temperature Tc, and are paramagnetic (disordering of the moments) above the temperature.
Related term paramagnetic material, diamagnetic material, antiferromagnetic material, magnetic domain
27-4. magnetic material
A material that is magnetized when placed in a magnetic field. "Magnetic materials" are classified into ferromagnetic material, paramagnetic material, diamagnetic material and antiferromagnetic material, from the viewpoint of how the material is magnetized.
Related term ferromagnetic material, paramagnetic material, diamagnetic material, antiferromagnetic material
27-5. paramagnetic material
A material that is magnetized in the direction of a magnetic field when placed in the magnetic field, and demagnetized when the magnetic field is removed.
Related term ferromagnetic material, diamagnetic material, antiferromagnetic material
28. real space
The space in which we exist. In a TEM, the "real space" includes the space of an object to be observed and the space where an image of the object is formed (image space).
Related term reciprocal space
29. real lattice
"Real lattice" is a set of points which have an infinitely regular arrangement in the real space and are arranged to be equivalent when viewed from every point. When a structure unit is given to each lattice point, a crystal is formed.
Related term reciprocal lattice
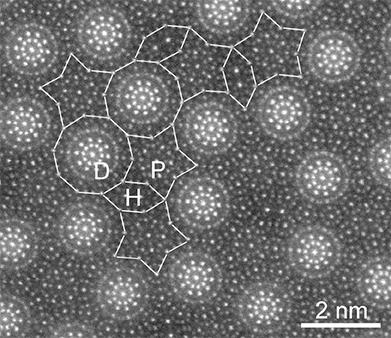
30. quasicrystal
"Quasicrystal" takes a new ordered structure which has no periodicity but a long-range order with bond orientational order between the constituent atoms. Thus, the quasicrystal is totally different from amorphous. Quasicrystals have been found in many alloys composed of aluminum and two transition metals exhibiting ferromagnetic properties.
HAADF-STEM image of an Al-Mn-Pd two-dimensional quasicrystal taken with a JEM-ARM200F at an accelerating voltage of 200 kV.
In the image, decagonal (D) structure units, star-shaped pentagonal (P) units and hexagonal (H) units are clearly seen. All the D units are joined by edge-sharing linkage, and the gaps between those D units are completely filled with P units and H units. (cf. JEOL News Vol. 50, p25 (2015))
Courtesy of the image: Professor Emeritus K. Hiraga, Tohoku University
31. zone axis
In a crystal, a group of planes parallel to a certain direction is called a crystal zone, and this direction is termed a "(crystal) zone axis."
32. paraelectric material
"Paraelectric material" generates dielectric polarizations when an electric field is applied to the material and the material looses the polarizations when the electric field is removed. In "paraelectric materials," three kinds of polarizations exist: (1) electronic polarization, (2) ionic polarization, and (3) orientational polarization. In electronic polarization, electrons are displaced against the atomic nucleus. In ionic polarization, positive ions are displaced against negative ions. In orientational polarization, molecules having permanent dipole moments change their directions under an electric field. The paraelectric material has a small permittivity and a small dielectric loss. A material which is called quantum paraelectrics does not transform to a ferroelectric phase but remains at the paraelectric state even around absolute zero temperature due to zero-point oscillation of phonons.
Related term ferromagnetic material, antiferroelectric material
33. precipitate
"Precipitate" is a solid formed as a new stable phase separated from the matrix crystal by atoms excessively dissolved in a supersaturated solid solution. When the degree of supersaturation of the dissolved atoms is low, they do not form a phase but are collected on grain boundaries or dislocations.
34. stacking fault
A kind of planar lattice defect (planar fault). When considering a perfect crystal to be produced by periodic stacking of (different kinds of) atomic planes, "stacking fault" means a fault where the order is violated.
Related term boundary, lattice defect, atomic plane
35. twins
When two adjacent crystals are symmetric with each other about a specific plane or a specific axis, these two crystals are called "twins."
36. phase transformation
A phenomenon where a substance transforms to a different state by a change of the external conditions (temperature, pressure, magnetic field, etc.). "Phase transformation" is classified into the first-order and the second-order phase transformations. The former exhibits a discontinuity in the first derivative of the free energy with respect to temperature, which includes melting of solids and vaporization of liquids. The latter exhibits a discontinuity in the second derivative of the free energy temperature, which includes the transformation of the ferromagnetic state (iron, etc.) to the paramagnetic state at Curie temperature and the transformation of the alloys from a chemical order state to a disorder state (and vice versa).
Related term chemical order/disorder
37. residence time
"Residence time" is a time when a particular compound or element spends in a system, or a time when a reactant spends in a process vessel or contacts a catalyst.
38.Polycrystal
A crystal composed of crystalline particles with different crystal orientations.
Related term single crystal
39. single crystal
39-1. single crystal
The single crystal is a crystal in which the constituent unit cells are aligned in the same orientation. A large single crystal is obtained under a specific growth condition.
Related term polycrystal
39-2. lanthanum hexaboride single-crystal tip
A tip used as a thermoelectron source. A lanthanum hexaboride (LaB6) single crystal, which is sharpened to a cone shape, is used. The LaB6 tip is indirectly heated at about 1800 K. Its brightness is 5×106 A/cm2.sr at 200 kV. The size of the crossover is ~10 μm. The energy spread of the emitted electrons from the filament is ~2 eV.
Related term hairpin filament, field-emission electron gun, FEG
40. unit cell
The minimum unit of the periodicity of a crystal. The size and form of the unit cell is defined by six lattice parameters or constants (a, b, c, α, β, γ).
Related term crystal structure, lattice parameter (constant)
41. short-range order parameter
In the case of a binary alloy, "short-range order parameter" means a probability of existence of atom B around atom A. It can be measured from the intensity of diffuse scattering that appears around a reflection specific to the ordered phase.
Related term long-range order parameter, ordered and disordered structure
42. Substitution
42-1. substitution
"Substitution" means that an atom or a group of atoms in a crystal or a molecule is substituted with other atom or other group of atoms.
42-2. substitutional atom
When the other kind of atom (solute atom) occupies an atom position instead of an original atom, the solute atom is termed a "substitutional atom."
42-3. freeze substitution
Freeze substitution is a technique to replace amorphous ice with an organic solvent (acetone, etc.) (dehydration) in a biological specimen fixed by rapid freeze fixation, where the specimen is subject to rapid freeze fixation (high pressure freezing or metal mirror freezing (slam freezing)). To prevent destruction of fine structures of the specimen, the substitution is carried out by raising temperature step-by-step from -80 ℃ to 4 °C over a few days. In the course of the substitution, chemical fixation and electron staining are often performed by adding osmium tetroxide and/or uranium acetate. Then, the substituted specimen is returned to room temperature and is subject to resin embedding. A TEM specimen is made by ultrathin sectioning the specimen.
Related term rapid freeze fixation, metal mirror freezing (slam freezing), high pressure freezing, amorphous ice
43. centro-symmetry
"Centro-symmetry" means that a crystal is symmetric with respect to a certain point.
44. long-range order parameter
In the case of binary alloys, a degree of order of atom species A and B increases with decreasing temperature after a substance undergoes a transition from a disordered phase to an ordered phase. The "long-range order parameter" means this degree of order. It can be measured from the intensity change of a specific reflection that appears in an ordered phase.
Related term short-range order parameter, ordered and disordered structure
45. superlattice
"Superlattice" is formed, for example, when a structural modulation occurs on a crystal structure to have a longer period (normally an integral multiple of the original period) than that of the original crystal structure. Such a modulation can be formed in a low-temperature phase due to phase transformation. An artificial superlattice is produced as a long-period multi-layer structure which is composed of more than two kinds of layer crystals deposited by molecular beam epitaxy (MBE). The superlattice structures are used for quantum-well laser diodes, high-temperature superconductive materials, and magnetic materials.
46. long period structure
For example, in the phase of CuAuⅠ, five ordered lattices are aligned in a direction followed by another five ordered lattices with an anti-phase shift to the former lattices, resulting into formation of a structure with a unit of 10 times larger than the original lattice. Such a structure is called a "long period structure." The phase CuAuⅠ appears in a narrow temperature range between the ordered phase and the disordered phase. In a diffraction pattern, the reflections from the ordered phase split to diffraction spots appearing at the positions corresponding to a period of 10 times that of the ordered phase. Long period structures appear in intermetallic compounds and SiC.
Related term ordered and disordered structure, anti-phase boundary
47. dislocation
47-1. dislocation
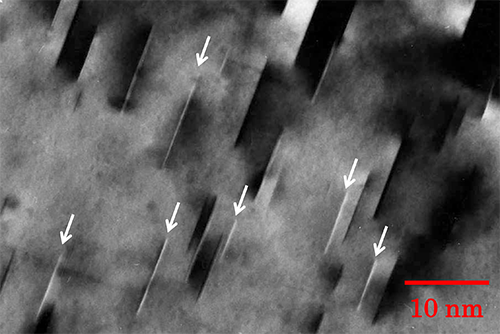
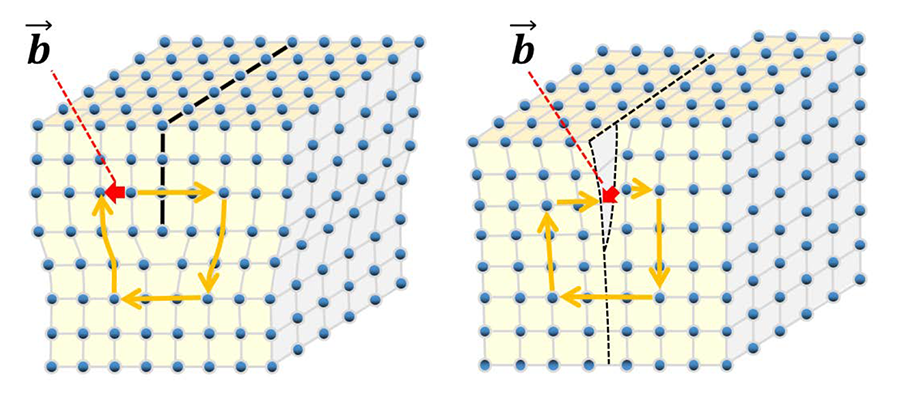
"Dislocation" is a linear or one dimensional defect in a crystal. When a part in a crystal is displaced against the other part, the line at which the displacement is starting is called a dislocation or a dislocation line. There are two types of dislocations, or edge dislocations and screw dislocations. The crystal around the dislocation is highly strained. Plasticity of a crystal is explained by motion and multiplication of dislocations. Fig. 1(a) schematically shows the atomic arrangement near an edge dislocation of a cubic crystal. An extra lattice plane (called an extra half plane) is shown by a black line. The end line of the extra half plane is the edge dislocation or edge dislocation line. Suppose a circuit along the crystal lattice around the dislocation line (Burgers circuit: indicated by yellow lines with allows), the end of the circuit after one round does not come back to the original lattice point but comes to the next lattice point. This displacement is termed the Burgers vector (indicated by a red allow), which characterizes the direction and amount of the atomic displacement caused by the dislocation. The Burgers vector (shown by a red line) of the edge dislocation is perpendicular to the dislocation line. Fig. 1(b) schematically shows the atomic arrangement near a screw dislocation. The screw dislocation (line) exists at the start (or end) line of the displacement and runs from the top to the bottom of the figure. The Burgers circuit in this case is seen to be spiral. The Burgers vector of the screw dislocation is parallel to the dislocation line (indicated by a red allow). Fig. 2 shows a bright-field image of dislocations in a silicon crystal. The dislocations (lines) are seen to be dark (black) lines because the regions near the dislocations come into a Bragg reflection. Fig. 3 shows a [001] atomic-resolution dark-field STEM image of a small-angle grain boundary of SrTiO3. Edge dislocations are seen to be arranged along the grain boundary, creating a small orientation change between both sides of the crystal.Related term.
Fig. 1.(a) Schematic of edge dislocation. (b) Schematic of screw dislocation.
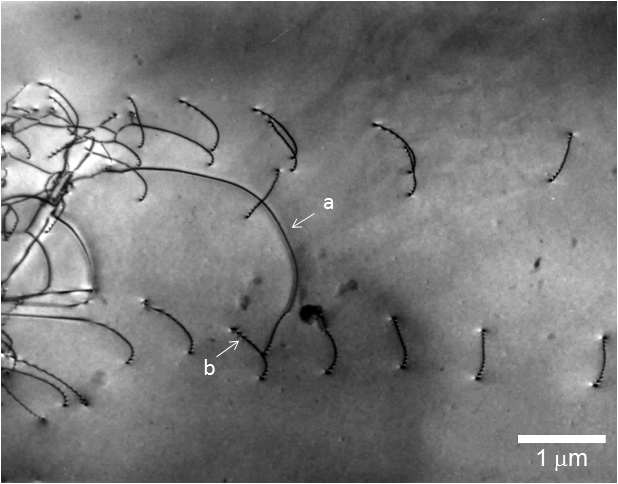 Fig. 2. Bright-field image of dislocations in a silicon crystal taken at an accelerating voltage of 200 kV.
(a) A black line (indicated by arrow "a") shows a dislocation line running parallel to the specimen surface.
(b) A black zigzag line (indicated by arrow "b") exhibits a dislocation running oblique to the specimen surface. The zigzag contrast is created by a dynamical diffraction effect.
Fig. 2. Bright-field image of dislocations in a silicon crystal taken at an accelerating voltage of 200 kV.
(a) A black line (indicated by arrow "a") shows a dislocation line running parallel to the specimen surface.
(b) A black zigzag line (indicated by arrow "b") exhibits a dislocation running oblique to the specimen surface. The zigzag contrast is created by a dynamical diffraction effect.
Fig. 3 (a) [001] atomic-resolution dark-field STEM image of dislocations on a small-angle grain boundary of SrTiO3 taken at an accelerating voltage of 200 kV. (Specimen courtesy: Prof. Y. Ikuhara, The University of Tokyo) (b) Edge dislocations are clearly visualized by selectively displaying crystalline planes along the grain boundary. (c) Schematic of the edge dislocation (gray circles indicate atomic columns).
Related term lattice defect, weak-beam method, partial dislocation, stacking fault
47-2. dislocation loop
When oversaturated point defects (vacancies or interstitials) accumulate in a plate form, a closed dislocation is produced at the edge of the plate. The closed dislocation is called "dislocation loop." In the case of the vacancy plate, when the atomic planes neighboring to the vacancy plate collapse to retrieve the inherent atomic distance, a stacking fault is produced. Whether the dislocation loop is the vacancy type or the interstitial type is determined by examining whether the dislocation image is formed inside or outside of the dislocation for the diffraction conditions of the positive and negative deviations from the Bragg condition.
47-3. imperfect dislocation
Related term partial dislocation
47-4. partial dislocation
The magnitude of Burgers vector b of a (perfect) dislocation is defined as the distance from a lattice point to the nearest lattice point. There may exist a meta-stable position for an atom given by a vector b1 whose magnitude is smaller than b. The Burgers vector of the perfect dislocation can split to b = b1+b2. The defects having vectors b1 and b2 are called "partial dislocation." Since the dislocation energy is proportional to the square of the Burgers vector, the splitting (extension) of the perfect dislocation is possible. However, a stacking fault is introduced between two partial dislocations. The energy of the stacking fault determines whether or not the extended dislocation occurs and the width of the stacking fault. When the splitting distance of the two partials is short, the use of the weak-beam method is effective for determining this splitting.
Related term dislocation, stacking fault
48. crystallographic point group
In crystal symmetry, all the combinations of symmetries that exist with respect to a fixed point in a crystal are called "crystallographic point groups." Symmetry elements consist of rotation(1-, 2-, 3-, 4- and 6-fold), rotary inversion(4-), mirror(m) and inversion(i). 32 independent point groups are constructed by the combinations of the 8 symmetry elements.
Related term rotation axis, rotary-inversion axis, mirror plane, inversion center, crystallographic space group, convergent-beam electron diffraction, CBED
49. point defect
"Point defect" is defined as atomic-size defect. In particular, when no atom exists at a lattice point, this defect is called "vacancy type point defect." On the other hand, when an atom is introduced into a position other than a lattice point, the defect is termed "interstitial type point defect." Vacancies can exist in a crystal at the thermal equilibrium state and play an important role for diffusion of atoms. Interstitial atoms are normally impurity atoms, but they can be the same atoms of the crystal when the atoms at the lattice points are ejected by irradiating the crystal with high-energy particles. In the latter case, since the interstitial atoms are easy to move, the atoms are likely to disappear by recombination with vacancies.
50. Isotropy
50-1. isotropy
"Isotropy" means that physical properties of a crystalline substance do not change regardless of its crystal orientations.
50-2. anisotropy
"Anisotropy" means that physical properties of a crystalline substance are different depending on its crystal orientations.
51. Neel wall
"Neel wall" is one type of the boundary structure of magnetic domains whose magnetization directions are antiparallel or different by 180°to each other. This wall is formed in a thin-film specimen. The magnetic dipoles parallel to the specimen surface continuously rotate in planes parallel to the surface at the magnetic wall, and connect to those of the adjacent domain with the opposite magnetization. In a diffraction pattern formed by two adjacent magnetic domains containing a Neel wall, a diffuse intensity arc appears connecting the diffraction spots from the two domains.
Related term Bloch wall, Lorentz electron microscopy
52. inversion center
A symmetry element of the crystallographic point group. An operation that transforms a coordinate (x, y, z) into (-x, -y, -z) (that is, the signs of the coordinates of each point are changed) is called the inversion operation. When this symmetry is operated for a crystal, the crystal can be equivalent to that before the operation. In this case, the point leaving fixed by the operation is called "inversion center."
Related term crystallographic point group, rotation axis, rotary-inversion axis, mirror plane
53. inversion domain boundary
"Inversion domain boundary" separates two adjacent crystals which coincide with each other by an inversion operation.
Related term boundary
54. amorphous
54-1. amorphou
A solid substance in which the arrangement of atoms and/or molecules is irregular and disordered.
54-2. amorphous ice
Amorphous ice means ice that does not take a crystalline state. When water is cooled below the melting point, ice crystal is formed and grows until its temperature reaches the re-crystallization temperature. Since formation of ice crystals can cause destruction of fine structures of a biological specimen, amorphous-ice formation without formation of ice crystals is required when rapid freeze fixation is applied. Thus, ice-crystal formation should be suppressed by cooling the specimen as rapid as possible down to the re-crystallization temperature.
Related term rapid freeze fixation, high pressure freezing, freeze substitution, metal mirror freezing (slam freezing), cryo-electron microscopy, ice embedding
55. composite crystal
A "composite crystal" is composed of two or more crystals whose structures have incommensurate periods (not expressed by an integral ratio) to each other.
Related term incommensurate structure
56. Impurity
56-1. impurity
"Impurities" are defined as substances contained in a pure substance. When a trace amount of impurities are doped into a semiconductor, energy levels characteristic of impurities are created in the band gap, from which electrons are excited to the conduction band or to which electrons are accepted from the valence band.
56-2. impurity atom
Foreign atoms mixed in a crystal, which are different kind from constituent atoms of the crystal. An "impurity atom" causes point defect, which is one of lattice defect.
Related term point defect
56-3.impurity level
"Impurity level" is defined as an energy level created in the band gap in a semiconductor, which is introduced by impurity atoms.
Related term cathodoluminescence
57. incommensurate structure
"Incommensurate structure" is defined as a structure where the periodicity of a structure arising from phase transformation cannot be expressed by a simple integral ratio with respect to the periodicity of the basic structure before phase transformation.
Related term superlattice, phase transformation
58. Bloch wall
"Bloch wall" is one type of the boundary structure of magnetic domains whose magnetization directions are antiparallel or are different by 180°to each other. The magnetic dipoles continuously rotate in planes parallel to the magnetic boundary and finally connect to those at the adjacent magnetic domain with opposite magnetization. This structure is formed in a bulk specimen with a thickness of more than 100 nm. The thickness of the Bloch wall is about 50 nm for iron. In a diffraction pattern formed by two adjacent magnetic domains containing a Bloch wall, a diffuse intensity line appears connecting the diffraction spots from the two domains.
Related term Neel wall, Lorentz electron microscopy
59. ferroelectric domain
The ferroelectric material has spontaneous electric polarizations under no external electric field. A "ferroelectric domain" means a region where the directions of all the polarizations are the same.
Related term ferromagnetic material
60. displacement
In crystallography, "displacement" means atomic displacement of an atom from a lattice point due to dislocation or stacking fault, and atomic displacement of an atom arising from (crystal) structural change at phase transformation.
61. deformation
"Deformation" means changes in shape or size of a substance due to contraction or expansion arising from changes of stress, temperature or chemical condition.
62. segregation
A phenomenon where the impurities or constituent elements in a metal or an alloy become unevenly distributed. "Segregation" often occurs when coagulation of alloy occurs.
63. modulated crystal
A "modulated crystal" takes an additional modulation wave of atomic displacement or composition to the fundamental structure, whose wavelength is a non-integral number of the period of the fundamental structure. The modulation provides satellite reflections around the fundamental reflections. The features of the satellite reflections are different depending on the modulation to be a transverse wave type or a longitudinal wave type.
Related term incommensurate structure, superlattice, phase transformation
64. Miller index
"Miller index (h, k, l)" specifies the lattice plane of a crystal, which is given by a/u, b/v, c/w, where u, v and w are the intercepts of the lattice plane on the crystal axes or coordinates at which the lattice plane intersects the crystal axes, and a, b and c are the unit cell dimensions of the crystal. If any of the ratios (a/u, b/v, c/w) take fractional number, the elements of the Miller index or the ratios are rewritten by integral number.
Related term lattice plane
65. dielectric material
"Dielectric material" is equivalent to "insulator." In a dielectric material, no free electrons exist or all electrons are bound to atoms or molecules. When an electric field is applied to a dielectric material, no electric current flows because of no free electrons in the material, but positive and negative charges are created on one surface and the other surface due to dielectric polarization, electric energy being stored.
Related term ferromagnetic material, paraelectric material, antiferroelectric material, metal
66. dielectric constant
A quantity to express the degree of polarizability of a material when a voltage is applied to. Or a quantity to express the amount of electrical energy to be stored in a material.
67. screw axis
A symmetry element of the crystallographic space group. When the whole crystal is rotated by a certain angle(180°, 120°, 90°, 60°) with respect to an axis and successively translated along the axis by a length of 1/2, 1/3, 1/4 or 1/6 of the crystal unit-cell, the translated crystal can be equivalent to the original crystal. In this case, this axis is called "screw axis." The screw axes are denoted by 21,31,32,41,42,43,61,62,63,64,65.
Related term crystallographic space group, glide plane